Post-doctoral scientist in PITOR, Paris, France
Pediatric Nephrologist in Necker-Enfants Malades, Paris, France
Design and protocol of an international multicentre study on molecular profiling of allograft rejection in paediatric kidney transplant patients: the PANDA-Kids-ATLAS study
Evgenia Preka1,2, Valentin Goutaudier1, Marion Rabant3, Camille Nicolas Frank4, Burkhard Tönshoff5, Anne-Laure Sellier6, Busutti Marco7, Fabian Eibensteiner8, Alexander Fichtner5, Patricia Costa Reis9, Antonia Bouts10, Jon Jin Kim11, Stephen Marks12, Costanza Pucci2, Marcus Weitz13, Jerome Harambat14, Marc Fila15, Maud Racape1, Fariza Mezine1, Jessy Dagobert1, Julien Hogan16, Isa Ashoor4, Olivia Boyer2, Alexandre Loupy1.
1Paris Institute for Transplantation and Organ Regeneration PITOR, Paris, France; 2Pediatric Nephrology, Necker-Enfants Malades Hospital, Paris, France; 3Department of Pathology, Necker-Enfants Malades Hospital, Paris, France; 4Division of Nephrology, Department of Pediatrics, Boston Children's Hospital, Boston, United States; 5Pediatric Nephrology, University Children's Hospital, Heidelberg, Germany; 6Pediatric Nephrology, Hôpital Femme Mère Enfant, Lyon, France; 7Division of Nephrology, Bambino Gesù Children's Hospital, Rome, Italy; 8Division of Paediatric Nephrology and Gastroenterology, Comprehensive Center for Pediatrics, Medical University of Vienna, Vienna, Austria; 9Pediatrics Department, Hospital de Santa Maria, Lisbon, Portugal; 10Pediatric Nephrology, Emma Children's Hospital, Amsterdam, Netherlands; 11University of Cambridge, Cambridge, United Kingdom; 12Pediatric Nephrology, Great Ormond Street Hospital for Children NHS Foundation Trust , London, United Kingdom; 13Department of General Pediatrics and Haematology/Oncology, University Children's Hospital Tuebingen, Tuebingen, Germany; 14Department of Pediatrics, Pediatric Nephrology Unit, Bordeaux University Hospital, Bordeaux, France; 15Pediatric Nephrology Unit, Centre Hospitalier Universitaire Arnaud de Villeneuve-Université de Montpellier, Montpellier, France; 16Pediatric Nephrology Department, Robert Debré Hospital, Paris, France
Introduction: Early and precise identification of kidney allograft rejection in paediatric kidney transplant (KT) recipients remains a critical unmet need. The PANDA-Kids-ATLAS study (Precision Allograft rejection using Novel Diagnostic Approaches in Kidney transplantation – Allograft Transcriptomics Landscape Analysis using Sequencing) is an international, multicentre cohort designed to validate rejection-related transcriptomic signatures and associate them with allograft outcomes. Here we present its design and protocol.
Methods: Clinical and biopsy data from paediatric (< 20 years old) KT recipients (2014–2028) across nine European and North American transplant centres will be collected retrospectively and prospectively. A total of 600 kidney allograft biopsies, including protocol and indication samples, will be analysed across diverse diagnostic and ethnic groups to ensure biological heterogeneity and robust molecular classifier validation. Formalin-fixed paraffin-embedded allograft biopsies will undergo central review by two Banff expert nephropathologists alongside interpretation using the Banff automation tool, following the Banff 2022 classification. A multidimensional transcriptomic approach will integrate molecular, clinical, biological, immunological, and histological data. RNA isolation, hybridization, and transcriptomic analysis will be performed using the NanoString nCounter® Bruker platform, and data normalization using housekeeping genes. The Banff Human Organ Transplant (B-HOT) panel will be used, a 770-gene panel designed to assess immune activation, inflammation, and tissue injury in allograft rejection. Quality control metrics will ensure data accuracy and molecular classifiers will be developed and validated based on discrimination, calibration, and overall model performance.
Results: The study design is detailed in Figure 1. A secure RedCap database has been created storing pseudo-anonymised clinical and molecular data, with direct web-based entry and storage on a private server. Samples transported to the Paris Transplant Group laboratory receive a lab number, linked to hospital records only at local sites, ensuring patient confidentiality. Ethics approval and data transfer agreements are being processed. To date, 91 pediatric KT patients (59.3% male, 40,7% female) have contributed to biopsies spanning various histological diagnoses. Thirty-one biopsies have been sequenced using the NanoString nCounter platform, generating transcriptomic data for in-house bioinformatics analysis. Patient recruitment remains open until December 2027, allowing additional centres to participate.
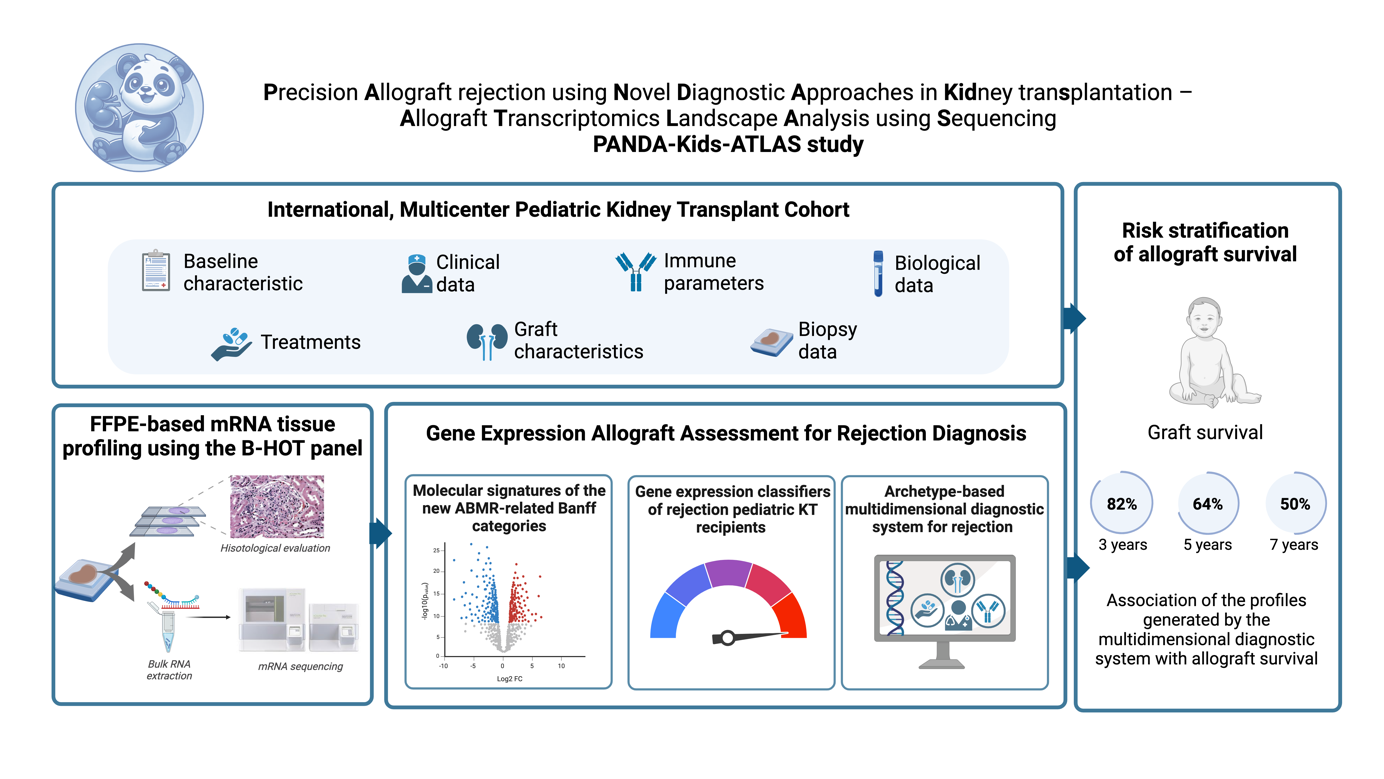
Conclusion: The PANDA-Kids-ATLAS study is a unique, international, multidimensional transcriptomic study studying molecular classifiers in paediatric allograft rejection. Findings are expected to provide pathophysiological insights, refine personalised treatment strategies, and enhance risk stratification for paediatric allograft rejection.
[1] molecular transcriptomics
[2] kidney biopsy
[3] antibody-mediated rejection
[4] T-cell mediated rejection
[5] molecular-based prediction scores
[6] pediatric kidney transplantation
[7] Banff classification