Post-doctoral scientist in PITOR, Paris, France
Pediatric Nephrologist in Necker-Enfants Malades, Paris, France
Urinary and blood mRNA biomarkers for detecting kidney allograft rejection in adults (The KTD-Innov study: NCT03582436)
Evgenia Preka1,2, Valentin Goutaudier2, Maud Racapé2, Pierre-Antoine Gourraud3, Moglie Le Quintrec4, Emmanuel Morelon5, Lionel Couzi6, Carmen Lefaucheur7, Nassim Kamar9, Dany Anglicheau8, Sophie Brouard3, Alexandre Loupy2.
1Pediatric Nephrology, Necker-Enfants Malades Hospital, Paris, France; 2Paris Institute for Transplantation and Organ Regeneration (PITOR), Paris, France; 3Nantes University Hospital, Nantes, France; 4Montpellier University Hospital, Montpellier, France; 5Lyon University Hospital, Lyon, France; 6Bordeaux University Hospital, Bordeaux, France; 7Kidney Transplant Department, Saint-Louis Hospital, AP-HP, Paris, France; 8Necker Hospital, AP-HP, Paris, France; 9Toulouse University Hospital, Toulouse, France
Background and Aims: Blood and urinary mRNA biomarkers are non-invasive tools with potential for detecting kidney allograft rejection. However, their ability to provide added diagnostic value beyond current standard-of-care (SOC) patient monitoring remains unclear.
Methods: In this prospective study, adult kidney transplant recipients were enrolled across seven French referral centres between July 2018 and December 2019 (ClinicalTrials.gov, NCT03582436). During the first-year post-transplantation, we quantified 19 blood and 12 urinary mRNA biomarkers during kidney allograft biopsies, including both protocol-specified and clinically indicated procedures. Primary outcome was the occurrence of allograft rejection, including antibody-mediated rejection (AMR), T cell-mediated rejection (TCMR), and mixed rejection, classified according to the Banff 2019 criteria.
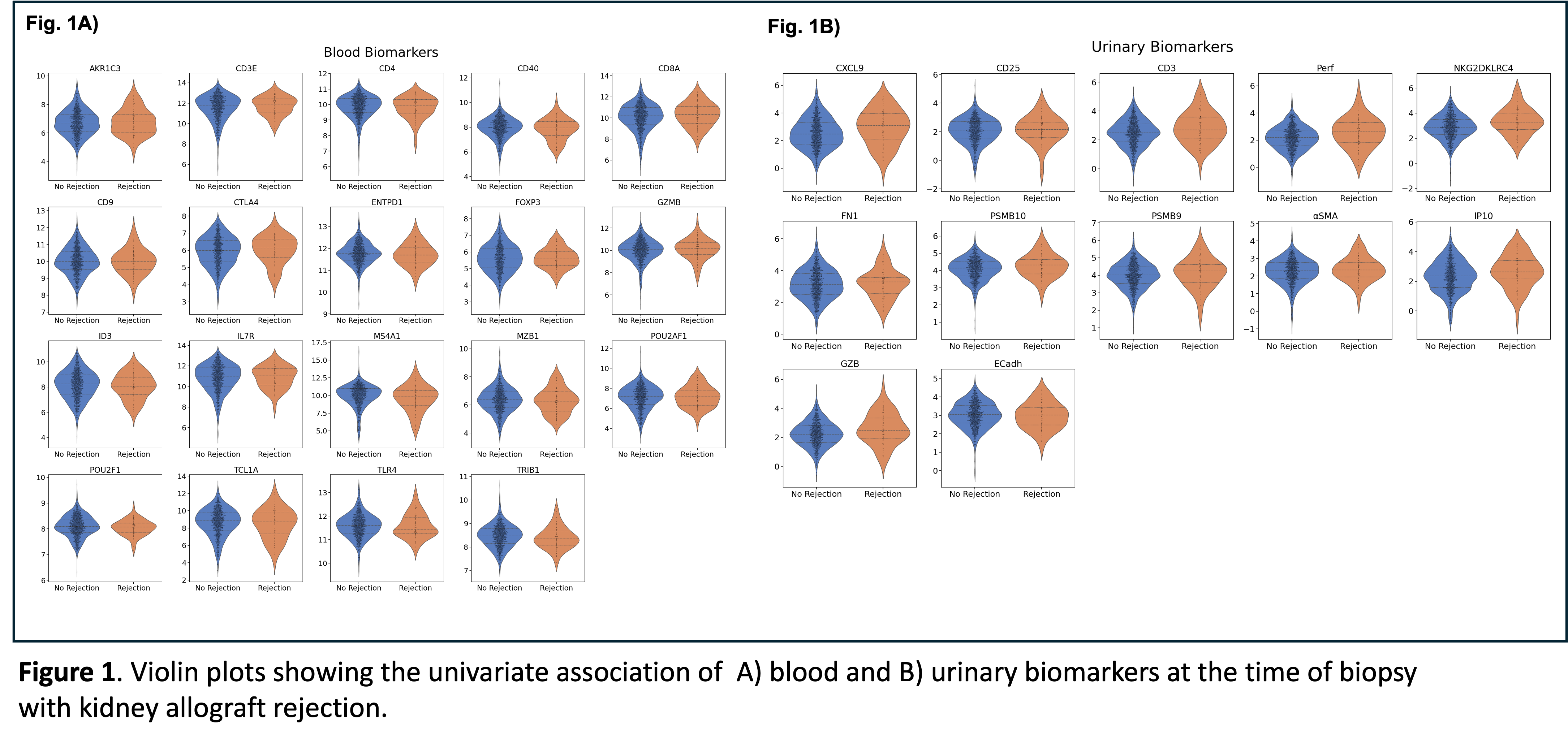
Results: Overall, 733 kidney transplant patients (64.1% male, 35.9% female) were included, with 1,549 biopsies paired with the mRNA biomarkers. The cumulative incidence of rejection was 9.7% (95% CI; 7.6%- 12.1%). None of the blood biomarkers tested (AKR1C3, CD3E, CD4, CD40, CD8A, CD9, CTLA4, ENTPD1, FOXP3, GZMB, ID3, IL7R, MS4A1, MZB1, POU2AF1, POU2F1, TCL1A, TLR4, and TRIB1) had a significant association with allograft rejection (Fig.1A). However, among the 12 urinary biomarkers (CXCL9, CD25, CD3, Perf, NKG2~4, FN1, PSMB10, PSMB9, αSMA , IP10, GZB, ECadh), eight-out-of-twelve, CXCL9 (p=0.001), CD3 (p=0.001), Perforin (p<0.001), NKG2~4 (p<0.001), PSMB10 (p=<0.001), PSMB9 (p=0.005), aSMA (p=0.043), IP10 (p=0.006) were significantly associated with allograft rejection (Fig.1B). Moreover, PSMB9 was the only urinary biomarker to show a discriminatory ability between TCMR and AMR (p=0.022, higher in TCMR). Despite these findings, integrating urinary biomarkers with SOC monitoring parameters failed to improve predictive accuracy compared to SOC monitoring.
Conclusion: Urinary mRNA—but not blood—biomarkers showed significant associations with kidney allograft rejection during the first-year post-transplantation. Nonetheless, these biomarkers did not improve predictive performance when added to existing SOC practices, underscoring the need for further research to optimise non-invasive diagnostic strategies.
The KTD-Innov study was funded by the French government, with financial support managed by the National Research Agency (ANR) under the program “Investissements d’avenir”, with the grant agreement no. ANR-17-RHUS-0010. INSERM-Action thématique incitative sur programme Avenir (ATIP-Avenir) provided financial support. VG received grants from the French-Speaking Society of Transplantation and the French Foundation for Medical Research. MS received a grant from Université Paris Cité. .
[1] blood biomarkers, urine biomarkers, kidney allograft rejection, kidney transplantation, gene expression, kidney biopsy