Biopsy findings from the VIRTUUS study by center surveillance practices: A North American cohort of incident kidney transplant recipients
Sandra Amaral1,10, Tom Blydt-Hansen2, Asha Moudgil3, Elizabeth Ingulli4, Paul Grimm5, Priya Verghese6, David Hooper7, Jodi Smith8, Debra Matossian6, Sarah Kizilbash9, Annie Chung1, Tran Bourgeois1, Jarcy Zee1,10, Patricia Weng11, Rachel Lestz12, Brendan Keating13, Juhi Kumar14.
1Pediatrics, Children's Hospital of Philadelphia, Philadelphia, PA, United States; 2Pediatrics, University of British Columbia, Vancouver, BC, Canada; 3Pediatrics, Children's National Hospital, Washington DC, DC, United States; 4Pediatrics, Rady Children's Hospital, San Diego, CA, United States; 5Pediatrics, Stanford University , Stanford, CA, United States; 6Pediatrics, Lurie Children's Hospital, Chicago, IL, United States; 7Pediatrics, Cincinnati Children's Hospital , Cincinnati , OH, United States; 8Pediatrics, Seattle Children's, Seattle, WA, United States; 9Pediatrics, University of Minnesota, Minneapolis, MN, United States; 10Biostatistics, Epidemiology, and Informatics, University of Pennsylvania, Philadelphia, PA, United States; 11Pediatric Nephrology, University of California, Los Angeles, CA, United States; 12Nephrology, Children's Hospital of Los Angeles, Los Angeles, CA, United States; 13Surgery, New York University Langone Health, New York City, NY, United States; 14Pediatric Nephrology, UPMC Children's Hospital of Pittsburgh, Pittsburgh, PA, United States
VIRTUUS Study Group.
Introduction: The utility of surveillance biopsies in pediatric kidney transplant remains controversial. Using the Validating Injury to the Renal Transplant Using Urinary Signatures (VIRTUUS) in Children Study Cohort, we compared biopsy findings between incident kidney transplant recipients at sites that perform surveillance biopsies (SB) vs. those doing only for-cause biopsies (FCB) to investigate differences in histopathology.
Methods: Eleven sites recruited incident kidney transplant recipients < 19 years old and contributed clinical and biopsy data for the first-year post-transplant. Biopsy data were adjudicated by two investigators using 2019 Banff criteria categories of normal, antibody-mediated changes, borderline and T-cell mediated rejection, interstitial fibrosis and tubular atrophy, and others. Descriptive statistics were used to describe histology between sites performing SB vs. FCB only. Generalized estimating equations were conducted to account for within-subject correlation and adjust for potential clustering effects across different centers when comparing differences in findings.
Results: 216 incident pediatric kidney transplant recipients underwent 387 kidney biopsies. Seven sites performed SB (63.6%). One hundred children (46.3%) had one, 65 (30.1%) had two and 51 (23.6%) had > 3 biopsies. Ninety-five participants (44%) showed rejection (Table 1).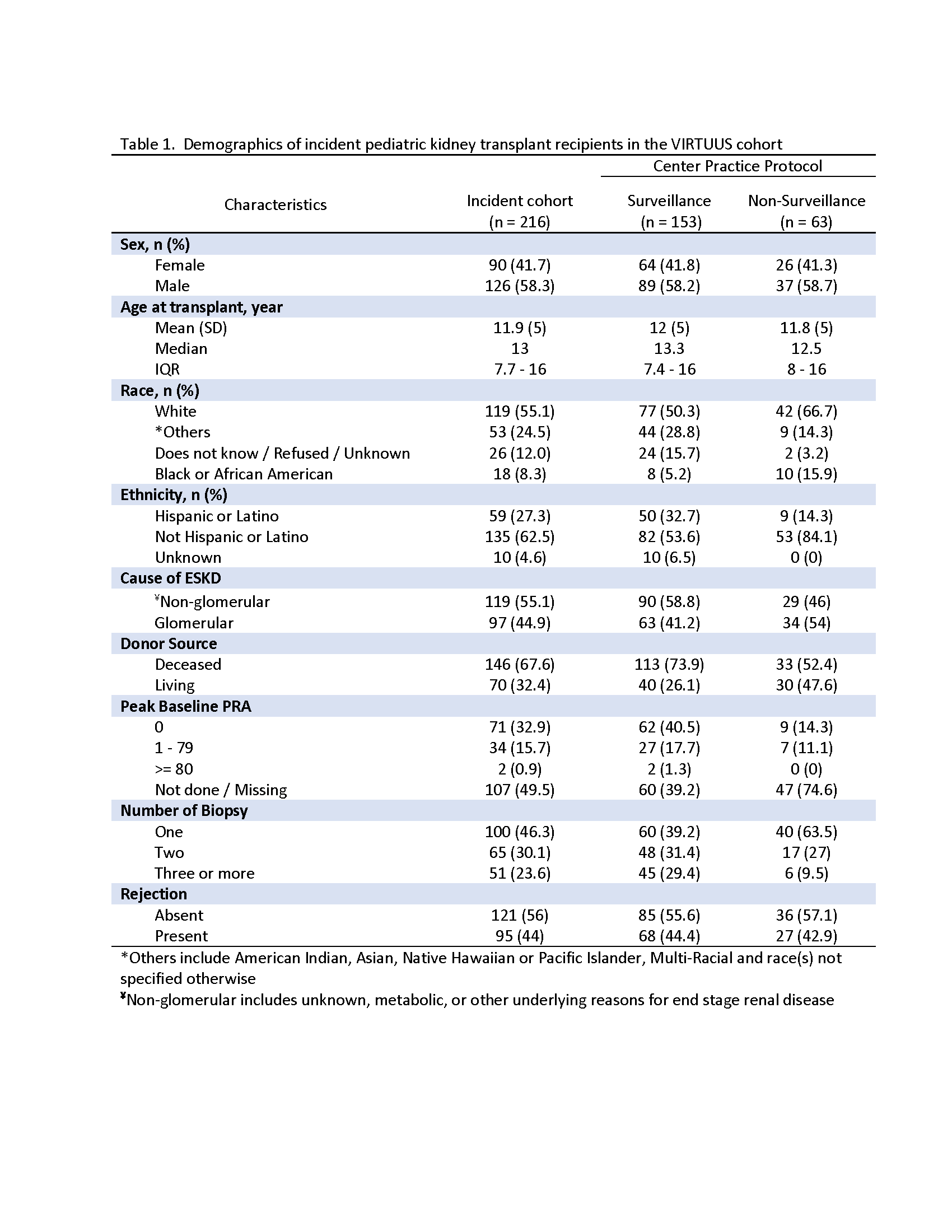 Biopsies from SB sites showed a wide range of abnormal pathology. FCB sites had significantly higher proportion of biopsies showing T-cell mediated rejection (23.1% vs. 10.1%, p<0.01) and “other findings” (51.7% vs. 32.1%, p=0.02). Most common causes of other changes in biopsies from FCB sites showed acute tubular injury (22%) and recurrent disease (14.3%) (Table 2).
Biopsies from SB sites showed a wide range of abnormal pathology. FCB sites had significantly higher proportion of biopsies showing T-cell mediated rejection (23.1% vs. 10.1%, p<0.01) and “other findings” (51.7% vs. 32.1%, p=0.02). Most common causes of other changes in biopsies from FCB sites showed acute tubular injury (22%) and recurrent disease (14.3%) (Table 2). 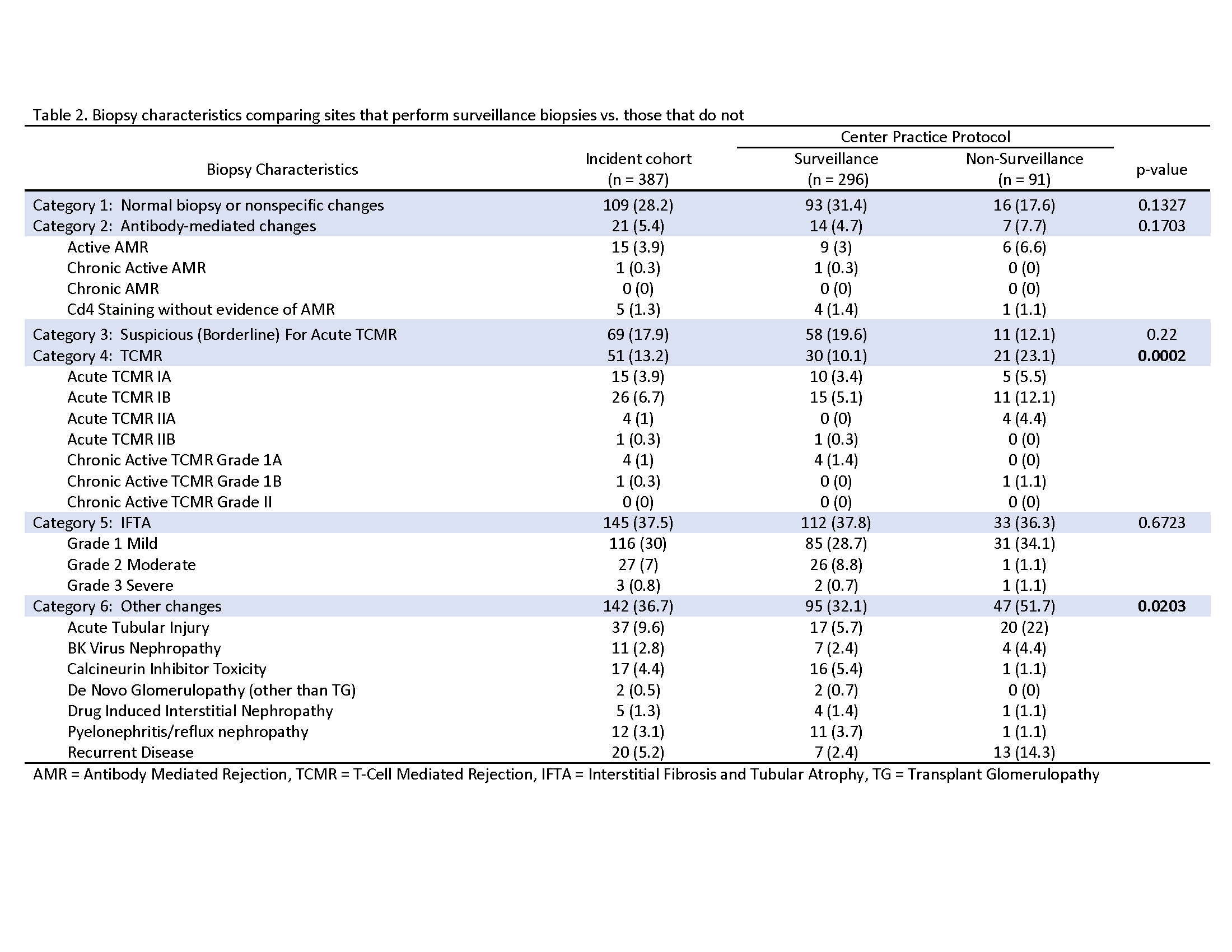
Conclusions: The VIRTUUS cohort represents a large diverse cohort of children with incident kidney transplants cared for in sites with varying biopsy practices. The prevalence and variety of abnormal histology differed between sites that performed SB vs. FCB only. Further analyses are underway to better characterize which findings were unexpected and actionable at the patient level.
Special thanks to all the patients who enrolled and to the study coordinators who worked tirelessly across the study period, which spanned the time of the pandemic..
References:
[1] kidney, transplant, children, biomarkers
[2] graft rejection, biopsies
