Evaluating feasibility and clinical relevance of pharmacogenomic testing using whole genome sequencing in pediatric kidney transplantation
Mercy Rophina1, Kiana Moi1, Annie Chung2, Brendan Keating1, Sandra Amaral2.
1NYU Langone Transplant Institute, NYU Langone Health, New York City, NY, United States; 2Pediatrics, Children's Hospital of Philadelphia, Philadelphia, PA, United States
Introduction: Pharmacogenomic (PGx) variability plays a critical role in drug response, efficacy, and toxicity, particularly in complex clinical settings like pediatric kidney transplantation. Individualized drug selection and dosing guided by genetic profiles can improve therapeutic outcomes and reduce adverse drug reactions. Whole genome sequencing (WGS) offers a comprehensive approach for PGx screening, enabling detection of common, rare, and complex structural variants. This study investigates the feasibility and clinical relevance of performing PGx analysis from WGS data in pediatric kidney transplant recipients.
Methods: Prevalent pediatric kidney transplant recipients were recruited to undergo WGS, performed by Illumina. Sequencing reads were aligned to the reference genome, and variant calling was carried out using the Illumina DRAGEN pipeline. Downstream analysis was performed using a custom bioinformatics and interpretation workflow restricted to a defined panel of genes and drug interactions with established clinical relevance. While analysis leveraged genome-wide data, only variants within specified pharmacogenes were evaluated. The pipeline supports detection of complex alleles, including copy number variants, hybrid alleles, and tandem arrangements in genes such as CYP2D6. Genes, alleles, and associated drug interactions were selected based on evidence from the U.S. Food and Drug Administration, Clinical Pharmacogenetics Implementation Consortium (CPIC), and Association for Molecular Pathology. Additionally, guidance from the American College of Medical Genetics and Genomics was incorporated to inform the interpretation and reporting of findings.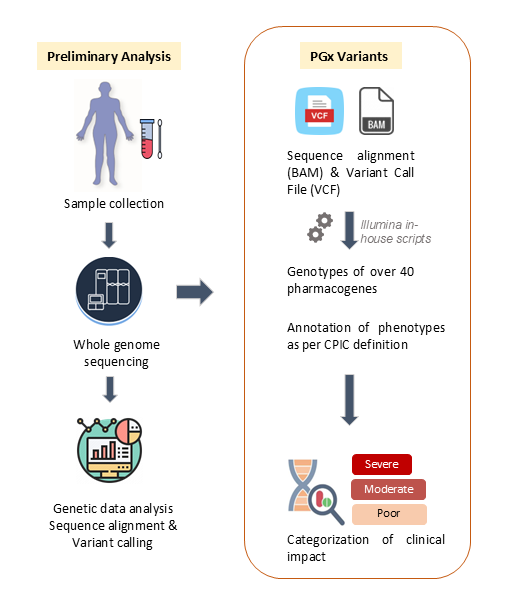
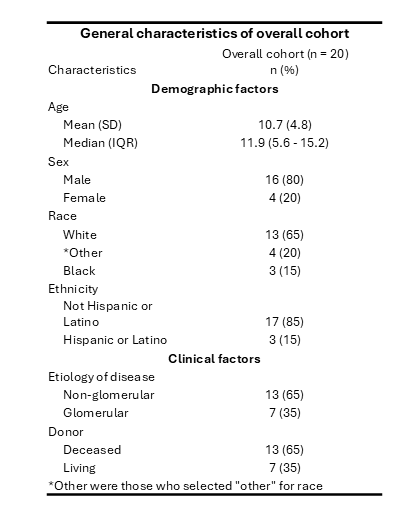
Results: 20 patients underwent WGS. Table 1 displays their clinical demographics
Among these 20 patients, a total of 196 pharmacogenomic variants were identified from 10 genes. Each variant corresponds to a CPIC guideline regarding medication management. A subset of these variants corresponds to a change from standard therapeutic recommendations. Notably, 40% of patients were CYP3A5 intermediate metabolizers, indicating a 1.5-2x increased starting dose for tacrolimus. 65% of patients had an actionable variant in CYP2C19, which impacts omeprazole metabolism. CYP2C19 ultrarapid metabolizers (n=3) and rapid metabolizers (n=1) suggest a 1.5-2x increased starting dose. For CYP2C19 intermediate metabolizers (n=9), a 50% reduced daily dose for therapy lasting >12 weeks is recommended.
Conclusions: This study demonstrates the feasibility of pharmacogenomic testing in pediatric kidney transplant recipients. A large number of pharmacogenomic variants with potentially actionable findings were identified. These results suggest PGx WGS is a potentially useful tool to inform a more tailored approach to pediatric kidney transplant management. Further analyses will examine whether a priori knowledge of these findings would have impacted treatment and outcomes.
References:
[1] pharmacogenomics
[2] kidney transplantation
