
Professor Stephen Marks, MD MSc MRCP DCH FRCPCH is Professor of Paediatric Nephrology and Transplantation at University College London Great Ormond Street Institute of Child Health and President of the British Association for Paediatric Nephrology. He is clinical lead for renal transplantation and Director of the National Institute for Health Research Great Ormond Street Hospital Clinical Research Facility at Great Ormond Street Hospital for Children NHS Foundation Trust. He has local, national and international roles including Chair of the Education Committee of the International Pediatric Transplant Association (IPTA) having also been an IPTA Councillor. His research continues to date in the fields of renal transplantation (including innovative drug trials concerning new anti-rejection therapies and assessment of children post-renal transplantation), systemic lupus erythematosus and vasculitis. He is on the editorial board for “Pediatric Nephrology” and “British Journal of Renal Medicine” and is associate editor for “Transplantation” and “Pediatric Transplantation”, which are the journals of The Transplantation Society (TTS) and the International Pediatric Transplant Association (IPTA), respectively.
Faecal microbiota transplantation for recurrent Clostridium difficile infection in paediatric kidney transplantation
Cansu Ceren Eryılmaz1,2, Zainab Arslan2, Garth Dixon3, James Hatcher3, Kelsey Jones4, Nigel Klein3, Delane Shingadia3, Jelena Stojanovic2, Sheila Boyle2, Stephen Marks2,5.
1Division of Paediatric Nephrology, Istanbul Faculty of Medicine, Istanbul, Turkey; 2Department of Paediatric Nephrology, Great Ormond Street Hospital for Children NHS Foundation Trust, London, United Kingdom; 3Department of Paediatric Infectious Diseases, Great Ormond Street Hospital for Children NHS Foundation Trust, London, United Kingdom; 4Deparment of Paediatric Gastroenterology, Great Ormond Street Hospital for Children NHS Foundation Trust, London, United Kingdom; 5NIHR Great Ormond Street Hospital Biomedical Research Centre, University College London Great Ormond Street Institute of Child Health, London, United Kingdom
Aims: Patients who have undergone renal transplantation (RTx) are immunosuppressed and more susceptible to developing Clostridium difficile infection (CDI), which can increase the risk of life-threatening sepsis and organ rejection. Faecal microbiota transplantation (FMT) is a highly effective treatment for CDI.
Methods: We present a 7-year-old boy with a history of posterior urethral valves (PUV) who underwent RTx followed by successful FMT.
Results: A two year old boy with chronic kidney disease secondary to congenital anomalies of the kidney and urinary tract due to posterior urethral valves and bilateral renal dysplasia underwent pre-emptive living related renal transplantation. He experienced three recurrences of CDI over five months from six years of age and was hospitalised due to abdominal pain and watery stools, requiring hydration and oral vancomycin treatment. He had positive Clostridium difficile (C. diff) PCR test and C. diff toxin A in three repeated analyses. Serologic tests for parasitic infections were negative. After an initial positive response to vancomycin treatment, the patient experienced repeated CDI and subsequently underwent a colonoscopy. The macroscopical and histopathological results were unremarkable, and inflammatory bowel disease and post-transplant lymphoproliferative disorder were ruled out. After multiple treatment regimens, including reducing vancomycin and adding fidaxomicin, FMT was proposed five years after RTx to eliminate C. diff from the patient's gastrointestinal tract.
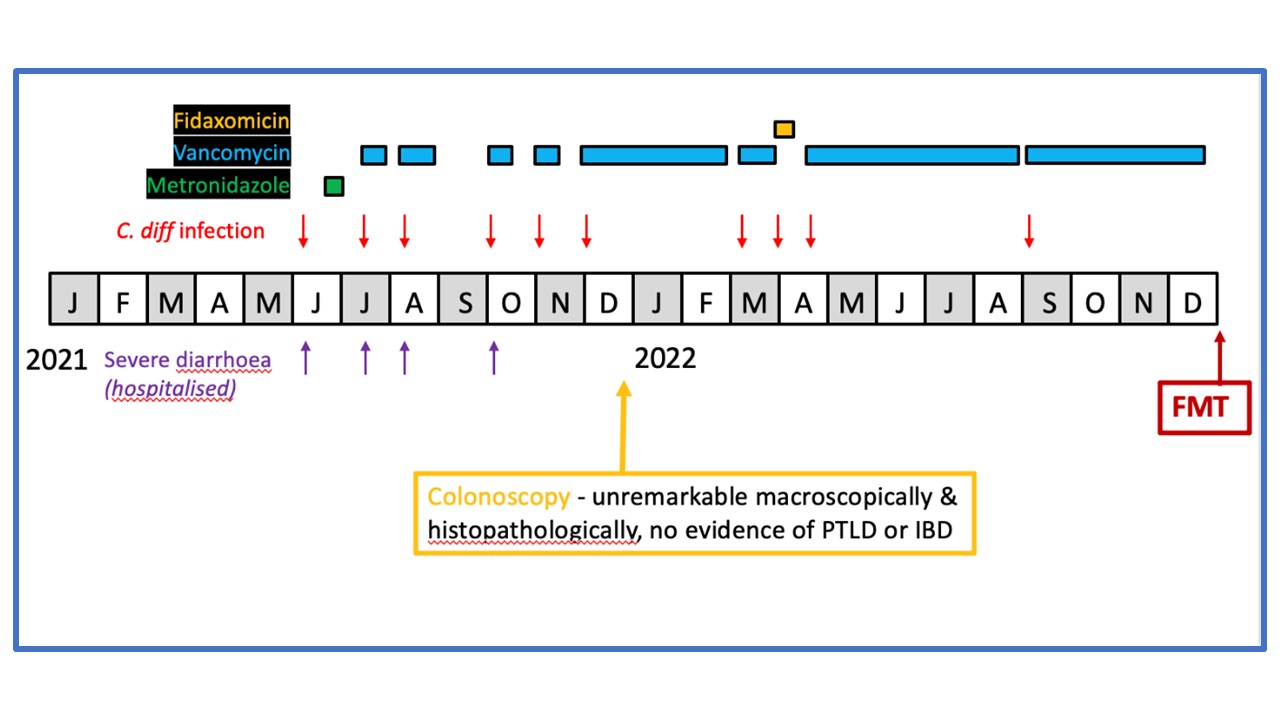
Donor faeces were collected in accordance with the regulations of the Human Tissue Authority. A successful FMT via colonoscopy under general anaesthesia was performed. No complications, fevers, adverse effects, or UTIs were observed post-procedure. During a 12-month follow-up after FMT, he remains clinically stable with stable renal allograft function with an estimated glomerular filtration rate of 42mls/min/1.73m2.
Conclusion: Children who have undergone RTx need to be closely monitored for recurrent CDI due to the increased risk of dehydration and renal allograft function deterioration. Although there are limited data regarding the effectiveness of new therapies for CDI, recent case reports indicate that FMT is a promising option for patients who have undergone RTx and are experiencing recurrent symptomatic episodes of CDI.
[1] Clostridium difficile infection
[2] oral vancomycin
[3] fidaxomicin
[4] faecal microbiota transplantation