Cedars-Sinai Medical Center
Alemtuzumab induction via intravenous vs. subcutaneous administration in pediatric kidney transplantation
Helen Pizzo1, Andrew Barbera2, Katherine Westreich3, Michael Seifert2, James Mirocha4, Kelly Kirshner1, Dechu Puliyanda1.
1Pediatric Nephrology, Cedars-Sinai Guerin Children's, Los Angeles, CA, United States; 2Pediatric Nephrology, University of Alabama at Birmingham , Birmingham, AL, United States; 3Pediatric Nephrology, University of North Carolina , Chapel Hill, NC, United States; 4Biostatistics Core, Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States
Background: Alemtuzumab is a lymphocyte-depleting induction agent used in kidney transplantation among high-risk highly sensitized recipients, or low-risk candidates on a steroid-withdrawal regimen. Although alemtuzumab is effective in preventing hyperacute rejection, it can result in prolonged lymphocyte depletion, which can be magnified with the routine use of antimetabolites and anti-infectives post-transplant. Here we assessed the dosing, tolerability, and outcomes in administering alemtuzumab induction via IV vs. SQ route in pediatric kidney transplant recipients.
Methods: A multicenter analysis of 119 patients <21 years old who received a kidney transplant and alemtuzumab induction was conducted. Dosing differences were evaluated by total dose and dose by body weight. Tolerability was defined by the development of leukopenia (WBC <3.0 x103/uL) or neutropenia (ANC <1.5 x103/uL); differences in total mycophenolate mofetil dose were also assessed. Outcomes in the first-year post-transplant were defined by eGFR, hospitalizations, bacterial infections, viral infections (BK, CMV, EBV), and rejection.
Results: 83 recipients received IV alemtuzumab compared to 36 who received SQ. Mean age at transplant was 12.5 yrs (standard deviation (SD) 6.0), with a slightly older population in the SQ group, 14.0 yrs (SD 5.5) vs. 11.9 yrs (SD 6.1) (P=0.07). More patients were highly sensitized (PRA >30%) in the SQ group, 14 (38.9%) vs. 10 (12.1%) (P=0.002). Total dose and dose by weight was higher in the SQ group, 22.3 mg (SD 8.9) vs. 14.9 mg (SD 9.5), and 0.57 mg/kg (SD 0.21) vs. 0.36 mg/kg (SD 0.18 mg), respectively. Mycophenolate dosing was higher in the IV group at 1-, 3-, 6-, and 12-months post-transplant; there were no differences in the incidence of leukopenia and neutropenia at the same time points, and eGFR remained similar between the groups (Table 1). There were no significant differences between the groups in the development of bacterial infections, viral infections (BK, CMV, EBV), hospitalization, or rejection in the first-year post-transplant.
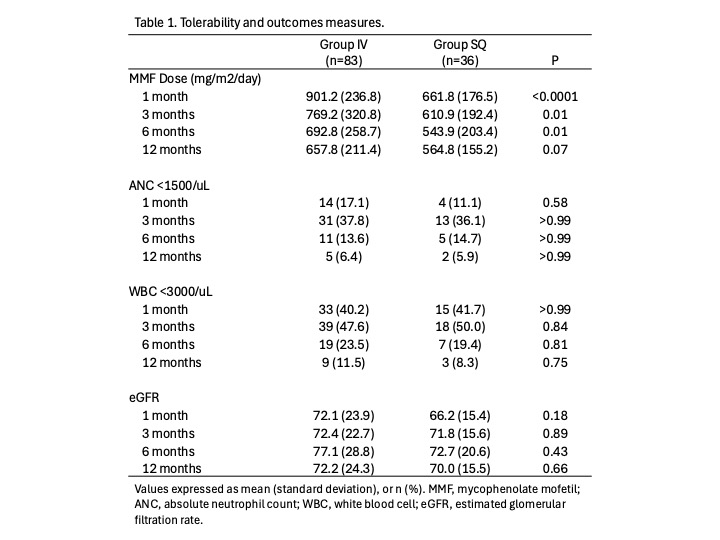
Conclusion: Despite the higher total and weight-based dose of alemtuzumab used in the SQ group, there were no differences in the measured outcomes, compared to IV alemtuzumab. Overall, alemtuzumab is well tolerated during the first-year post-transplant as an induction agent in the subsets of pediatric kidney transplant population in which it is used.
References:
[1] Immunosuppression
[2] Outcomes
[3] Infection
[4] Leukopenia
[5] Neutropenia
[6] rejection
Lectures by Helen Pizzo
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sun-21 09:15 - 10:45 |
Monitoring after pediatric kidney transplant | Risk factors for loss of varicella immunity after pediatric kidney transplantation | MOA 6 |
|
Sat-20 10:00 - 11:00 |
Optimizing outcomes after pediatric kidney transplantation | Alemtuzumab induction via intravenous vs. subcutaneous administration in pediatric kidney transplantation | MOA 6 |
|
Thu-18 17:00 - 18:00 |
Kidney Posters - from P1.1 to P1.32 | Low incidence of rejection and de novo donor-specific antibody formation following covid-19 vaccination or infection among pediatric kidney transplant recipients | MOA 10 (Exhibit Area) |
