Alejandro Zarauza Santoveña, Spain
Attending Physician
Pediatric Nephrology
Hospital Universitario La Paz
Adoptive cell therapy based on CD45RO+/RA- memory lymphocites infusions to treat BK virus-associated nefropathy in kidney transplant patients.
Alejandro Zarauza Santoveña1, Luisa Sisinni2, Karima Al-Akioui Sanz3, Mercedes Gasior Kabat4, Natalia Morales Capitán4, Laura García Espinosa1, Sara Rodríguez López1, Marta Melgosa Hijosa1, Antonio Pérez Martínez2,3.
1Pediatric Nephrology, Hospital Universitario La Paz, Madrid, Spain; 2Pediatric Onco-Hematology, Hospital Universitario La Paz, Madrid, Spain; 3IdiPAZ-CNIO Pediatric Onco-Hematology Clinical Research Unit, CIBERER-ISCIII, Madrid, Spain; 4Hematology and Cell Therapy , Hospital Universitario La Paz, Madrid, Spain
Introduction: BK virus associated nephropathy (BKvAN) is an important cause of graft dysfunction and loss in pediatric kidney transplant (KTx) recipients. There is no effective antiviral treatment, and reduction of immunosuppression (IS) is the first line of management. When it is not enough, renal prognosis is poor, and there is no evidence of the efficacy of other commonly used therapies (imTOR, IVIG).
Adoptive immunotherapy with donor memory lymphocytes infusions (mDLI) was previously used to treat viral infections in immunocompromised patients showing to be safe and potentially effective. We present a case of BKvAN in which the use of T lymphocytes (CD45RO+ /RA-) infusions containing specific memory T cells against BKV coming from haploidentical donor achieved control of the infection.
Methods: Donor selection is based on a good IFN-γ response against BKV peptide-pool, and on the partial or complete major histocompatibility system (HLA) donor-patient matching. Assessment of healthy donor included clinical parameters and HLA typing. A non-mobilized apheresis was performed using Spectra Optia® Apheresis System (Terumo BCT) followed by a an immunomagnetic CD45RA+ depletion (CliniMACS Plus device,Miltenyi Biotec®). CD45RA-depleted product was cryopreserved into several aliquots in a cell dose of 1 x106/Kg CD3+CD45RO+/RA- for later use.
Results: 3 year-old boy, ESKD due to posterior urethral valves, receives a first cadaveric KTx (6/6 HLA missmatches). Standard IS regime with basiliximab, steroids, Tacrolimus (TAC) and mophetil mycophenolate (MMF). No surgical complications, serum creatinine (SCr) at discharge 0,4 mg/dL.
BKv DNAemia was detected during routine follow up at +3 m. IS adjustment, adjuvant therapies, SCr and BKV DNAemia evolution are shown in figure 1.
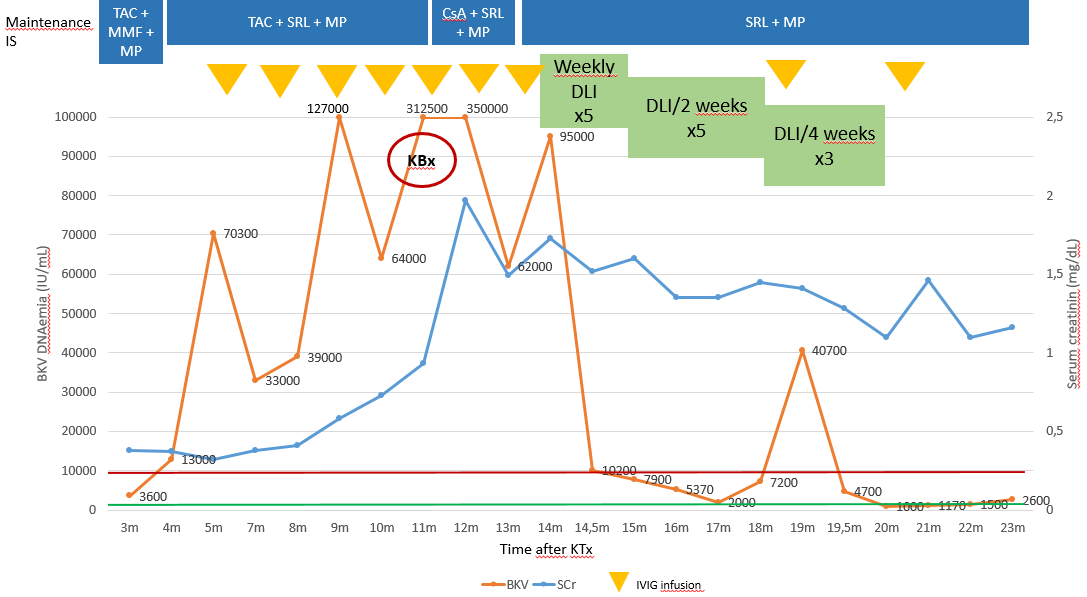
At +11m, despite the treatments given, BKV DNAemia remained high and renal function rapidly worsened, so a renal biopsy was performed, showing severe BKvAN with no signs of rejection. IS was minimized and the use of CD45RO+/RA- mDLI was considered. Patient’s mother, HLA haploidentical, was confirmed to be immunologically competent against BKv. A total of 13 aliquots were obtained, and administered in a schedule depending on viral load. mDLI were administered via peripheral venous line, under premedication (antihistamine), in a 10 min infusion. All the infusions were well tolerated without any adverse events.
After 13 aliquots administered, treatment was stopped. BKV DNAemia remains consistently under 10,000 IU/mL and kidney function is stable (SCr 1,1-1,4 mg/dL). Anti-HLA antibodies have always been negative and there has been no sign of rejection.
Conclusion: Adoptive immunotherapy based on mDLI of CD45RO+/RA- cells containing specific memory lymphocytes against different pathogens has demosntrated to be a safe therapy for immunocompromised patients with refractory viral infections. Here we showed that It may be a valuable option for the treatment of BKvAN.
References:
[1] BK virus
[2] Adoptive inmunotherapy
[3] Infection
[4] Kidney transplant
[5] Cell therapy
