Systematic review on the use of donor-derived cell-free deoxyribonucleic acid (ddcfDNA) for diagnosing rejection in paediatric solid organ transplant recipients
Rishil Patel1,2, Dinarda U Nadobudskaya1, Maya Banerjee3, Ali Aarif3, Hardya G Hikmahrachim4, Deborah Ridout5, Nithiakishna Selvathesan6, Stephen D Marks2,7.
1University College London Great Ormond Street Institute of Child Health, University College London, London, United Kingdom; 2Department of Paediatric Nephrology, Great Ormond Street Hospital for Children NHS Foundation Trust, London, United Kingdom; 3University College London Medical School, London, United Kingdom; 4Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia; 5Paediatric Epidemiology Biostatistics, University College London Institute of Child Health, London, United Kingdom; 6Department of Paediatric Nephrology, The Hospital for Sick Children, Toronto , ON, Canada; 7NIHR Great Ormond Street Hospital Biomedical Research Centre, University College London Great Ormond Street Institute of Child Health, London, United Kingdom
Background: Percutaneous biopsy remains the gold standard for detecting rejection in paediatric transplant recipients, yet its invasive nature poses substantial risks. Donor-derived cell-free deoxyribonucleic acid (ddcfDNA) has emerged as a promising biomarker that may allow clinicians to diagnose rejection episodes with fewer procedural complications.
Aim: To synthesise current evidence on the diagnostic accuracy of ddcfDNA for identifying rejection episodes in paediatric solid organ transplant recipients (SOTRs) and identify factors influencing its performance.
Methods: We conducted a comprehensive search (Cochrane, PubMed, Embase, and Web of Science) from database inception until 16 January 2023 for original studies assessing ddcfDNA to diagnose rejection in paediatric SOTRs. Eligible publications included any method of ddcfDNA extraction and quantification, with rejection confirmed by biopsy or clinical judgment. Risk of bias and applicability were appraised using QUADAS-2, and meta-analysis was carried out via the Rutter-Gatsonis model to summarise overall sensitivity and specificity estimates.
Result: Eight studies met the inclusion criteria, involving 734 paediatric recipients. All were judged to have high or unclear risk of bias in at least one QUADAS-2 domain. Across six studies (446 samples) comparing ddcfDNA with histologically confirmed rejection, pooled sensitivity was 78.54% (95% CI: 69.59–85.41%) and pooled specificity was 82.66% (95% CI: 68.07–91.42%) (Figure 1 and 2). Specificity was notably lower when ddcfDNA was quantified in whole blood rather than plasma, although sensitivity remained similar. No significant differences in diagnostic performance emerged when stratifying by clinical context (for-cause vs. mixed populations), organ type, or rejection subtype. In one liver transplantation study, ddcfDNA discriminated rejection better than standard aminotransferase levels; similarly, ddcfDNA outperformed serum creatinine in kidney transplant recipients in another study. The certainty of evidence was rated as low, reflecting methodological limitations (e.g., partial verification bias, unclear blinding). Heterogeneity was observed among the included studies, and although confidence intervals were wide, they did not cross equivalence lines. Deeks’ funnel plot test did not suggest publication bias (p>0.05).
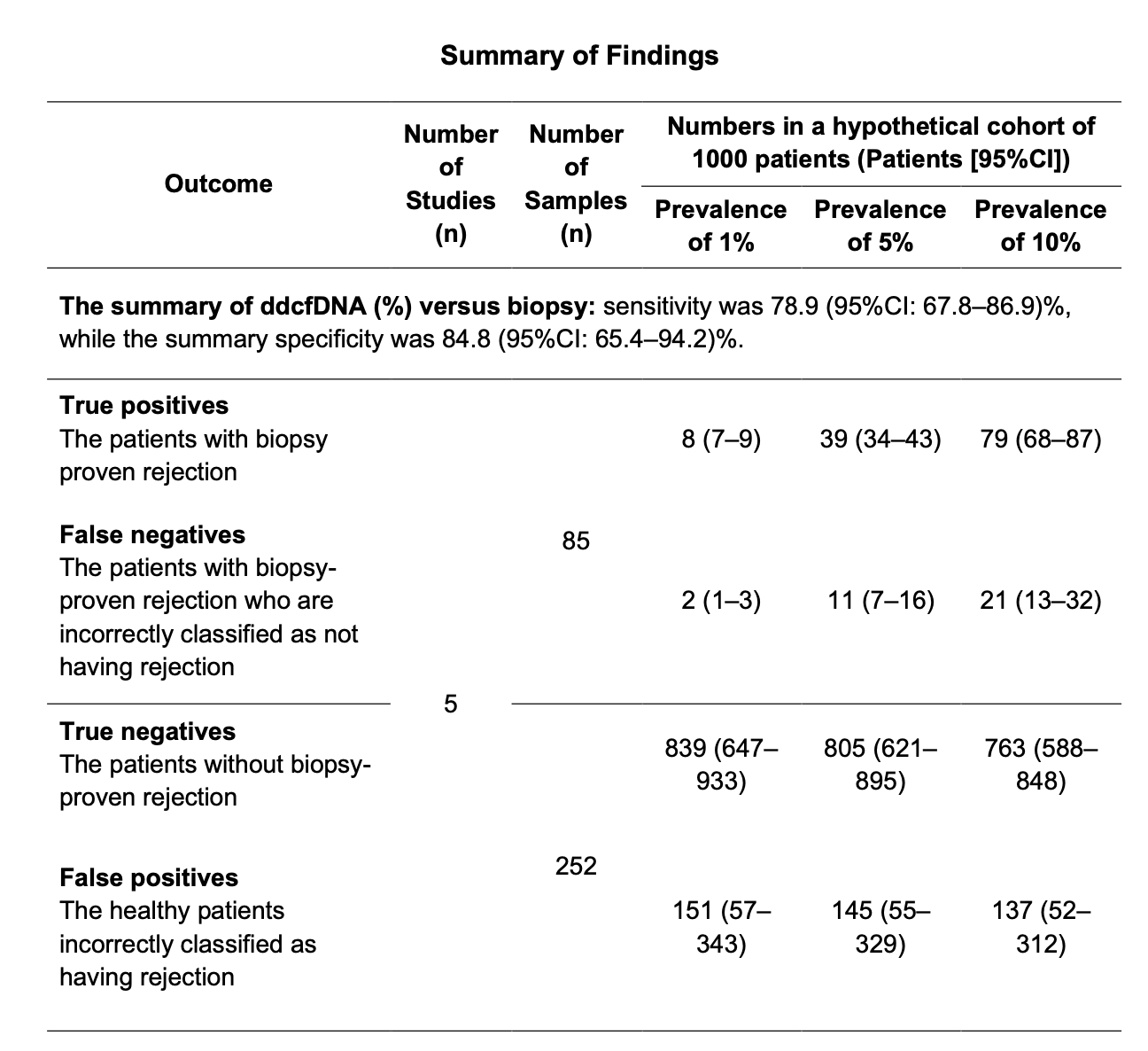
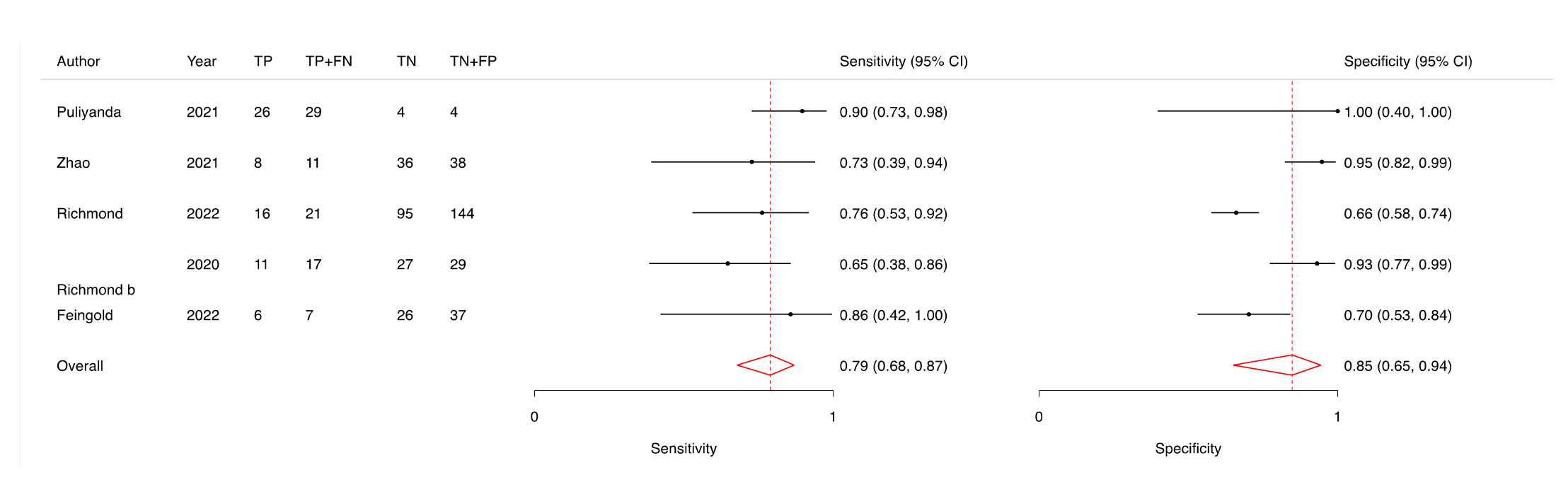
Conclusion: Low-certainty evidence suggests that ddcfDNA has moderate-to-high accuracy for identifying rejection in children with kidney, heart, liver, or lung transplants, with a pooled sensitivity of nearly 79% and specificity of approximately 83%. Although promising, the findings are tempered by study heterogeneity and limitations in reporting. Future research should validate the optimal sampling matrix (plasma vs. whole blood), refine test thresholds for clinical use, and explore cost-effectiveness, especially for centres weighing the potential of ddcfDNA as a routine surveillance tool or a targeted strategy in symptomatic patients.
In conducting this review, an author (D.U.N) received funding as a part of a Master’s scholarship from the Indonesian Endowment Fund for Education. The open-access publication was facilitated by the University College London through the Jisc agreement with Wiley. The funders did not have any role in determining the review methodology or manuscript writing. .
[1] systematic review
[2] diagnostic accuracy
[3] donor-derived cell-free DNA
[4] graft rejection
[5] solid organ transplant