Donor-derived cell-free DNA elevations with acute rejection types in a multicenter NAPRTCS registry cohort of children with kidney transplants
Vikas Dharnidharka1, Sara Boynton2, Dechu Puliyanda3, Olga Charnaya2, Priya Verghese4, Vaishali Singh5, Raoul Nelson6.
1Rutgers Robert Wood Johnson Medical School, New Brunswick, United States; 2Johns Hopkins Medical Center, Baltimore, United States; 3Cedars-Sinai Medical Center, Los Angeles, United States; 4Ann and Robert Lurie Children's Hospital, Chicago, United States; 5Medical College of Wisconsin, Milwaukee, United States; 6University of Utah, Salt Lake City, United States
NAPRTCS.
Introduction: Serum creatinine, the most common biomarker to detect acute rejection (AR), is neither sensitive enough nor is its rise early enough, to prevent significant acute rejection-induced damage. Peripheral blood donor-derived cell-free DNA (dd-cfDNA) percentage has been identified as a biomarker for allograft injury in adults, but studies in a large cohort of children are lacking. The North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS), a voluntary multi-center consortium that has been collecting data on children with kidney transplants since 1987, expanded the registry with a CareDx grant in 2022 to collect dd-cfDNA data.
Methods: In addition to transplant data routinely collected by NAPRTCS (e.g. recipient demographics, donor characteristics, immunosuppression and follow up graft function) dd-cfDNA results obtained as part of standard care, whether surveillance or for-cause, were collected. Centers provided the assay used (Allosure or Prospera or other), and whether the test was associated with a biopsy or an episode of AR.
Results: To date, we have collected 1386 dd-cfDNA percentage results, from 305 unique pediatric kidney transplant recipients (median 3 results per patient, IQR 2 - 6). Results were entered from test dates Oct 8th 2018 to February 2nd 2024, representing transplant dates Oct 8th 2008 to April 2nd 2024, from 14 different centers, median 16 patients and 58 results per center. In this analysis, we evaluated the subset with at least 2 dd-cfDNA levels with the same assay and clearly defined AR type on the biopsy or AR form (788 results from 191 unique patients, of whom 60% were male, 62% white, median age 11 years, 49% deceased donor). When comparing the 768 samples without AR to the 20 samples with AR, the dd-cfDNA showed a progression to higher median levels (0.27 in No rejection to 0.44 in Acute cellular rejection to 0.91 in Antibody-mediated rejection to 3.4% in Mixed rejection; Figure).
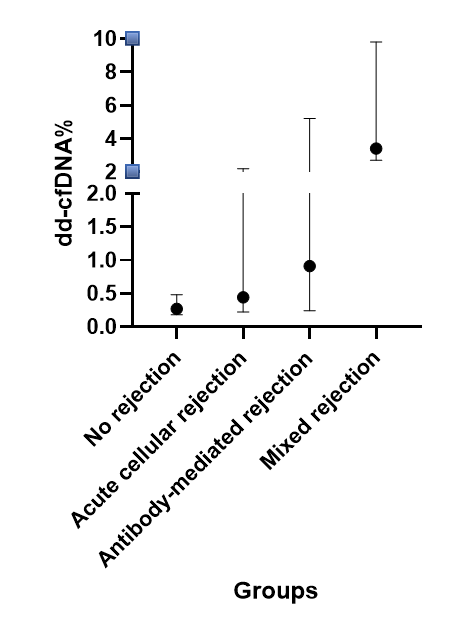
Conclusions: In this large pediatric cohort study, higher donor-derived cell-free DNA percentage results were associated with kidney transplant biopsy or an AR event, and levels were higher by type of AR.
Further analyses will allow us to calculate test efficacy for AR using receiver operating characteristic curves, separated by surveillance or for-cause detailed biopsy results.
CareDx for funding of the dd-cfDNA result data collection.
[1] Acute rejection
[2] Kidney transplant
[3] Biomarker