Atessa Bahadori, Canada has been granted the CareDx Congress Scientific Awards

Kidney-alone transplantation while on siRNA therapy for primary hyperoxaluria type 1
Atessa Bahadori1, Asma Nasir2, Nithiakishna Selvathesan1,6, Peter Schulga1,3, Douglas Stewart3, Vladimir Belostotsky4, Christopher T Chan2, Carmen Avila-Casado5, Elizabeth Harvey1,6, Armando Lorenzo7,8, Ashlene M McKay1,6, Istvan Mucsi2, Chia Wei Teoh1,6, Mathieu Lemaire1,6,9.
1Division of Nephrology, The Hospital for Sick Children, Toronto, ON, Canada; 2Department of Medicine (Nephrology), University of Toronto, Ajmera Transplant Centre, Toronto, ON, Canada; 3Renal Department, Royal Hospital for Children, Glasgow, United Kingdom; 4Division of Nephrology, Department of Pediatrics, McMaster Children’s Hospital, Hamilton, ON, Canada; 5Department of Laboratory Medicine & Pathophysiology, University of Toronto, Ajmera Transplant Centre, Toronto, ON, Canada; 6Department of Paediatrics, Faculty of Medicine, University of Toronto, Toronto, ON, Canada; 7Division of Urology, Department of Surgery, University of Toronto, Toronto, ON, Canada; 8Department of Surgery, Faculty of Medicine, University of Toronto, Toronto, ON, Canada; 9Cell Biology Program, SickKids Research Institute, Toronto, ON, Canada
Aims/Purpose: Primary hyperoxaluria type 1 (PH1) is a rare genetic disease characterized by the deposition of oxalate in end-organs, leading to kidney failure (KF) and systemic oxalosis. Current expert guidelines recommend liver-kidney transplantation without native nephrectomies for patients without a pyridoxine-sensitive AGXT variant. Historically, kidney-alone transplant (KATx) was considered a safe option only for patients responsive to pyridoxine supplementation. Many clinical trials have shown that small interfering Ribonucleic Acid (siRNA)-based therapy is an effective treatment for PH1. These siRNAs knock down critical enzymes involved in hepatic oxalate synthesis: lumasiran and nedosiran inhibit glycolate oxidase and lactate dehydrogenase, respectively. A few published reports detail nine PH1 patients with successful KATx while on siRNA therapy.
Methods
Case series.
Results: We present three additional PH1 patients who have undergone KATx under siRNa coverage. Patient 1 is the second reported case of an uneventful living-related KATx, under nedosiran coverage. At 24 months post-transplant, he has stable kidney function (serum creatinine of ~150 µmol/L) and plasma oxalate levels consistently below 6.0 µmol/L. Patient 2 (Figure 1) is a teenager who underwent diseased-donor KATx with an uncomplicated course for 10 months while on lumasiran. She then developed an acute kidney injury caused by three obstructive stones lodged at the uretero-ureteral anastomosis that originated from the ipsilateral native kidney. Following stone lithotripsy and stent insertion, allograft function recovered to baseline; elective unilateral native nephrectomy was performed 5 months later. Patient 3 (Figure 2) is a 6-month old who underwent an uneventful living-related KATx under lumasiran coverage at 3.25 years of age. Four months following KATx, graft function is good with persistently elevated urine oxalate-to-creatinine ratio.
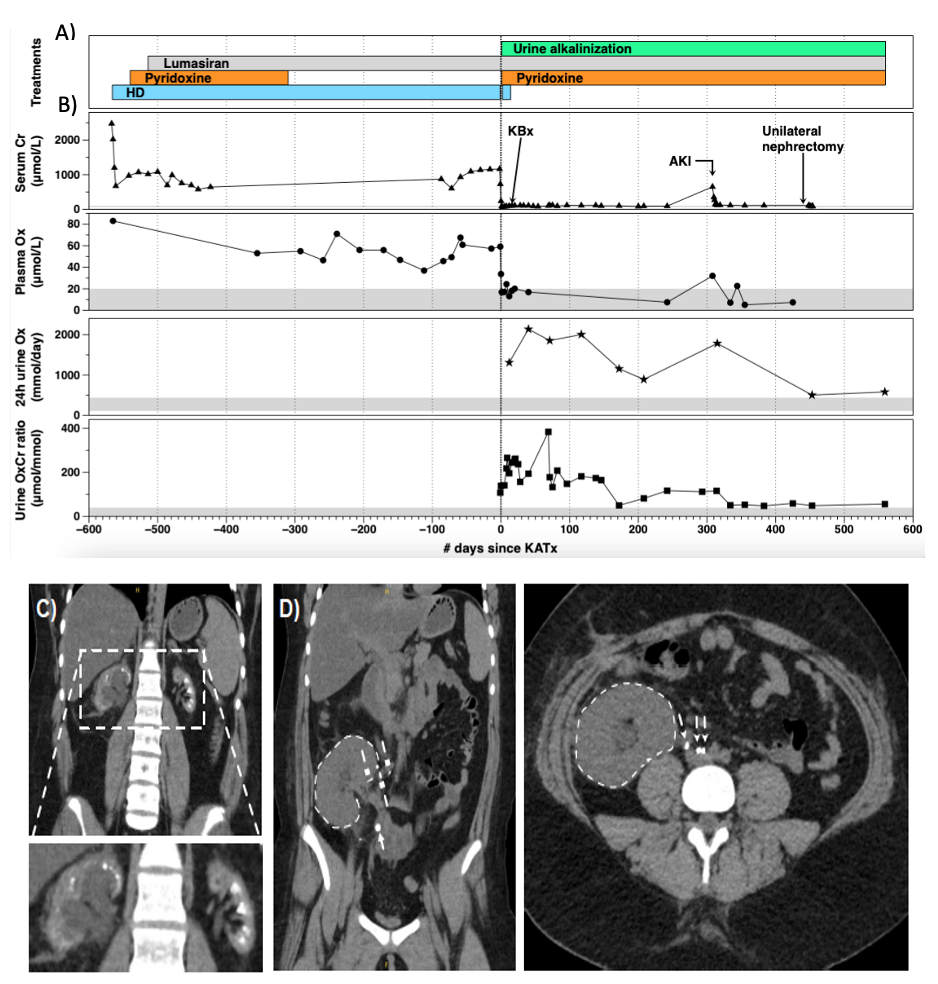
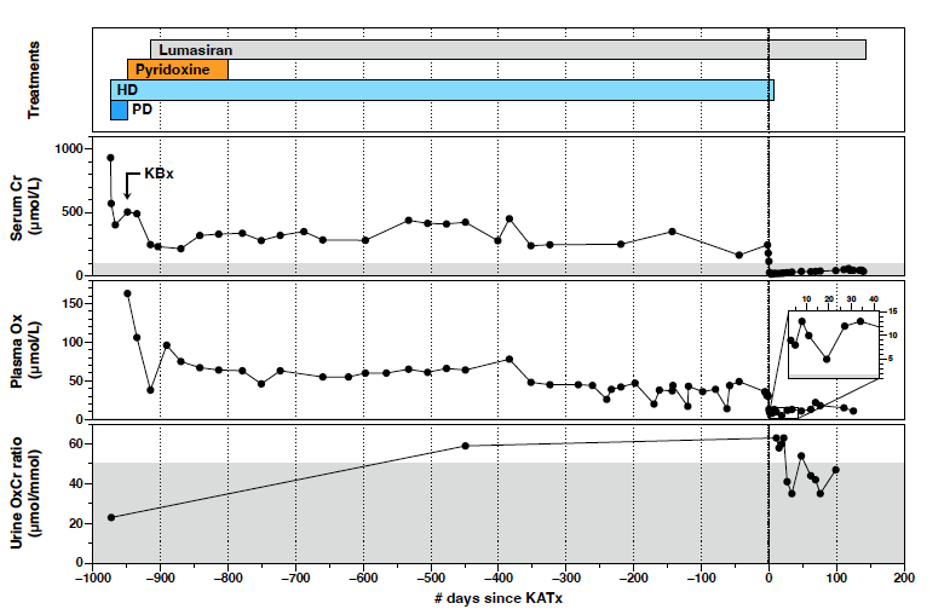
Conclusions: Our case series provides additional evidence of the short- and medium-term success of KATx in three PH1 patients treated with lumasiran or nedosiran. It also highlights the importance of long-term vigilance for obstructive nephrolithiasis caused by oxalate stones originating from the native kidneys. Ipsilateral nephrectomy should be considered if a uretero-ureteral anastomosis is chosen to leverage a normal ureterovesical junction.
Atessa Bahadori and Asma Nasir are first co-authors.
[1] Primary hyperoxaluria
[2] Kidney-alone transplantation
[3] Lumasiran, Nedosiran