European multi-center trial with imlifidase prior to kidney transplant in highly sensitized children
Gema Ariceta1, Lars Wennberg2, Timo Jahnukainen3, Julien Hogan4, Anna Runström5, Jan Tollemar5.
1Hospital of Vall d’Hebron, Paediatric Nephrology, Barcelona, Spain; 2Karolinska University Hospital, Department of Transplantation Surgery, Stockholm, Sweden; 3Helsinki University Hospital, HUS Children and Adolescents, Helsinki, Finland; 4Hopitâl Universitaire Robert Debré, Service de Néphrologie Pédiatrique, Paris, France; 5Hansa Biopharma AB, Clinical R&D, Lund, Sweden
Introduction: Imlifidase is an immunoglobulin G-cleaving enzyme of Streptococcus pyogenes, a cysteine protease that specifically inactivates all four human subclasses of soluble and membrane bound immunoglobulin G (IgG). Imlifidase reduces the load of donor-specific antibodies (DSAs) to a level enabling kidney transplantation. In Europe, imlifidase is conditionally approved for desensitization treatment of highly sensitized (HS) adult kidney transplant patients with a positive crossmatch against a deceased donor (Idefirix®, EU/EEA SmPC May-2024).
As in adults, sensitization of pediatric patients scheduled for transplantation is usually due to previous renal or other solid-organ transplants. Although sensitization can also occur as a result of blood transfusion, infections, or other immunizations. In pediatric patients receiving a first transplant, the majority of pediatric recipients have low or no panel reactive antibodies (PRA), whereas 3% have a PRA of more than 80%. To date, high levels of DSAs are if present a challenging barrier to transplantation in both adults and children.
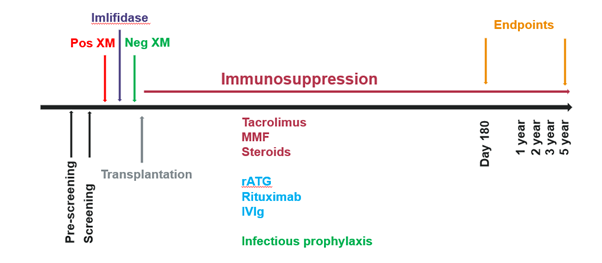
The aim is to present the EMA imlifidase pediatric investigational plan (PIP), a single-arm, multi-center trial to evaluate efficacy and safety of imlifidase in HS children (1-17 years) with end stage renal disease (ESRD), receiving a kidney transplant.
Methods: This European multi-center trial (5-10 centers, open for referrals) will investigate the efficacy and safety of imlifidase administered to 10 pediatric patients, 1-17 years old with ESRD, waiting for a kidney transplant. The trial will include HS pediatric patients (PRA ≥80%) who will receive and accept crossmatch positive kidney offers from either deceased or living donors (DD/LD, where imlifidase will convert a positive crossmatch to a negative. The primary objective is to evaluate crossmatch conversion within 24 hours of imlifidase treatment. The duration of the interventional trial period after transplantation will be 6 months for each patient. The trial also includes collection of pharmacokinetic (PK), pharmacodynamic (PD), efficacy and safety data up to 5 years after the transplantation (ClinicalTrials.gov, NCT05753930).
Conclusions: The completion of the trial is aimed at addressing PK, PD, safety and efficacy in the highly sensitized kidney transplant pediatric ESRD patient population, thus expanding the imlifidase indication to include children. The study sites are open for referrals and so far, two patients are waiting for an organ offer.
[1] Desensitization
[2] Kidney transplant
[3] Highly sensitized
[4] Donor-specific antibody (DSA)