Pharmacogenetics in pediatric kidney transplant: Leveraging CYP3A5-guided therapy for improved patient outcomes
Sierra Scodellaro1,2, Caoimhe Costigan3, Alexander Chung1, Chia Wei Teoh3,4, Ruud Verstegen1,4,5, Iris Cohn1,2,4.
1Division of Clinical Pharmacology and Toxicology, Department of Pediatrics, The Hospital for Sick Children, Toronto, ON, Canada; 2Program in Translational Medicine, The Hospital for Sick Children, Toronto, ON, Canada; 3Division of Nephrology, Department of Pediatrics, The Hospital for Sick Children, Toronto, ON, Canada; 4Department of Pediatrics, Faculty of Medicine, University of Toronto, Toronto, ON, Canada; 5Division of Rheumatology, Department of Pediatrics, The Hospital for Sick Children, Toronto, ON, Canada
Introduction: The success of kidney transplantation (KT) relies heavily on the ability to effectively manage pre- and post-transplant pharmacotherapy. Tacrolimus (TAC), a widely prescribed immunosuppressant to prevent organ rejection in KT, exhibits significant variability in therapeutic response due to genetic factors that impact TAC metabolism by CYP3A5. Pharmacogenetics (PGx) promotes individualized pharmacotherapy, yet this promising approach is not standard practice in the work-up or management of most individuals undergoing KT. This cross-sectional study evaluates the potential impact of PGx-guided pharmacotherapy in a cohort of pediatric KT patients. By demonstrating how PGx can optimize pharmacotherapy, especially TAC management, this research aims to inform larger-scale studies and enhance the application of precision medicine in KT.
Methods: Genotyping of nine genes (NUDT15, CYP2C19, CYP2C9, CYP2D6, CYP3A5, F5, SLCO1B1, TPMT, VKORC1) was completed in a cohort of 48 pediatric patients who were evaluated for KT. Clinical and demographic data were gathered by chart review via the electronic health record. Genotyping results were correlated with pre- and post- transplant medication history, especially focusing on TAC dosing and therapeutic drug monitoring. Median results are reported with interquartile range [IQR] 25-75%. Statistical analysis was performed using the Mann-Whitney U test.
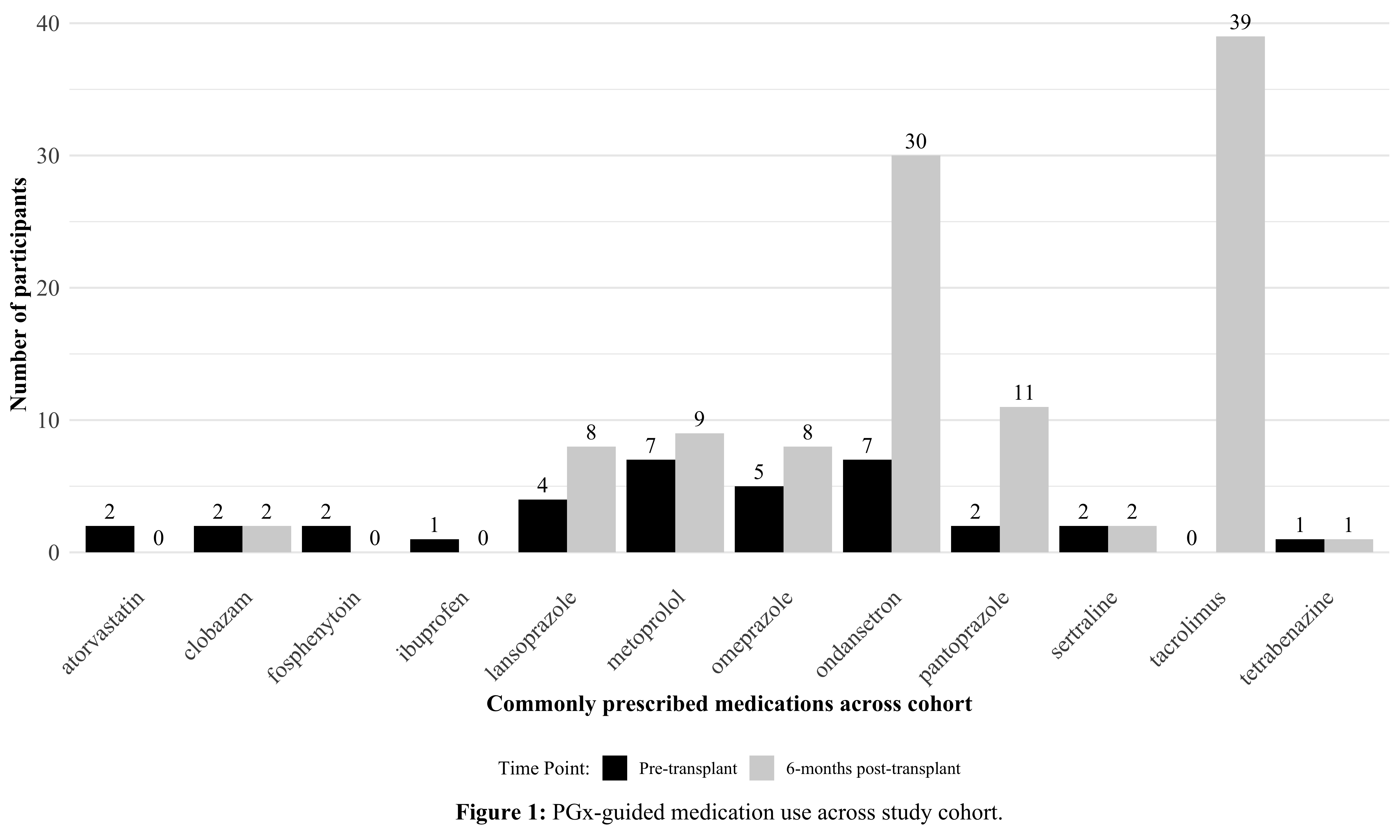
Results: Thirty-nine out of 48 participants underwent KT and completed 6 months follow-up. Medication usage within the total cohort revealed 12 commonly prescribed drugs, used both pre- and 6-months post-transplant, all of which are associated with established PGx guidelines; TAC, ondansetron and proton pump inhibitors being the most frequent (Figure 1). For 20 (42%) participants, PGx results would have warranted deviations to standard dosing for at least one medication that was prescribed throughout study participation. Twelve (25%) patients were predicted to benefit from increased TAC dosing (CYP3A5 expressors) compared to the average population (CYP3A5 non-expressors). Specifically, we demonstrated that median TAC dosing to achieve initial therapeutic levels was higher (0.48 [0.39 – 0.69] vs 0.28 [0.18 – 0.34]) mg/kg/dose, p < 0.001) in expressors (n = 9) compared to non-expressors (n = 30). Additionally, the median time to reach therapeutic levels was 10.00 [7.00 -18.00] and 5.00 [4.00 – 7.25] days for expressors and non-expressors, respectively (p = 0.002).
Conclusion: This study highlights potential applications of PGx-guided pharmacotherapy for patients undergoing KT. Specifically, the use of CYP3A5 genotype may be beneficial to guide individualized TAC dosing and reducing the overall time to achieve therapeutic TAC targets, which may impact graft function. Incorporating PGx data to optimize the use of immunosuppressants and other commonly used medications throughout KT, offers an approach to implement precision pharmacotherapy for this patient population.
[1] Pharmacogenetics
[2] Kidney transplant
[3] Tacrolimus
[4] CYP, cytochrome P450
[5] Precision medicine