Outcomes after basiliximab, thymoglobulin, or alemtuzumab induction for pediatric kidney transplant: A single center study
Jenny J Yan1, Lauren Walter2, Hannah Deckelbaum3, Daniel J Westreich4, Katherine D Westreich 3.
1PharmD Program, UNC Eshelman School of Pharmacy, Chapel Hill, NC, United States; 2Department of Pharmacy, UNC Medical Center, Chapel Hill, NC, United States; 3Division of Pediatric Nephrology, UNC School of Medicine, Chapel Hill, NC, United States; 4Department of Epidemiology, UNC Gillings School of Global Public Health , Chapel Hill, NC, United States
Introduction: Alemtuzumab is a lymphocyte depleting agent used as induction immunosuppression (IS) in pediatric kidney transplants, allowing a steroid-free maintenance IS regimen. Current research is limited regarding the long-term outcomes using this approach to induction IS in pediatric kidney transplant.1-3
Methods: We performed a single-center study evaluating short- and long-term outcomes of pediatric kidney transplants performed between May 2014 and May 2024. Recipients of their first kidney transplant were included in the analysis; patients who received a second transplant in that window were censored at time of second transplant. Prior to 2019, basiliximab or thymoglobulin were used as induction IS based on risk of rejection or recurrent autoimmune disease, followed by triple-drug maintenance IS with tacrolimus, mycophenolate, and prednisone. Beginning in 2019, alemtuzumab was used for induction for both low- and high-risk recipients aged 6 and up, followed by steroid-free, two-drug maintenance IS. We analyzed data using descriptive statistics and unadjusted Kaplan-Meier curves stratified by induction IS regimen.
Results: We studied 79 children for a median of 4.94 years (interquartile range [IQR] 2.33, 6.90) of follow-up after kidney transplant. In an unadjusted comparison, there was less rejection over time (p=0.03) among patients who received alemtuzumab (5.7% at 1 year, 45.3% at 5 years) as compared to a combined group of patients who received either basiliximab or thymoglobulin (13.7% at 1 year, 60.9% at 5 years). There were no significant differences in viral infections between induction strategies (Table 1). There was slightly higher risk of neutropenia among alemtuzumab recipients (Table 1) but the difference was not statistically significant.
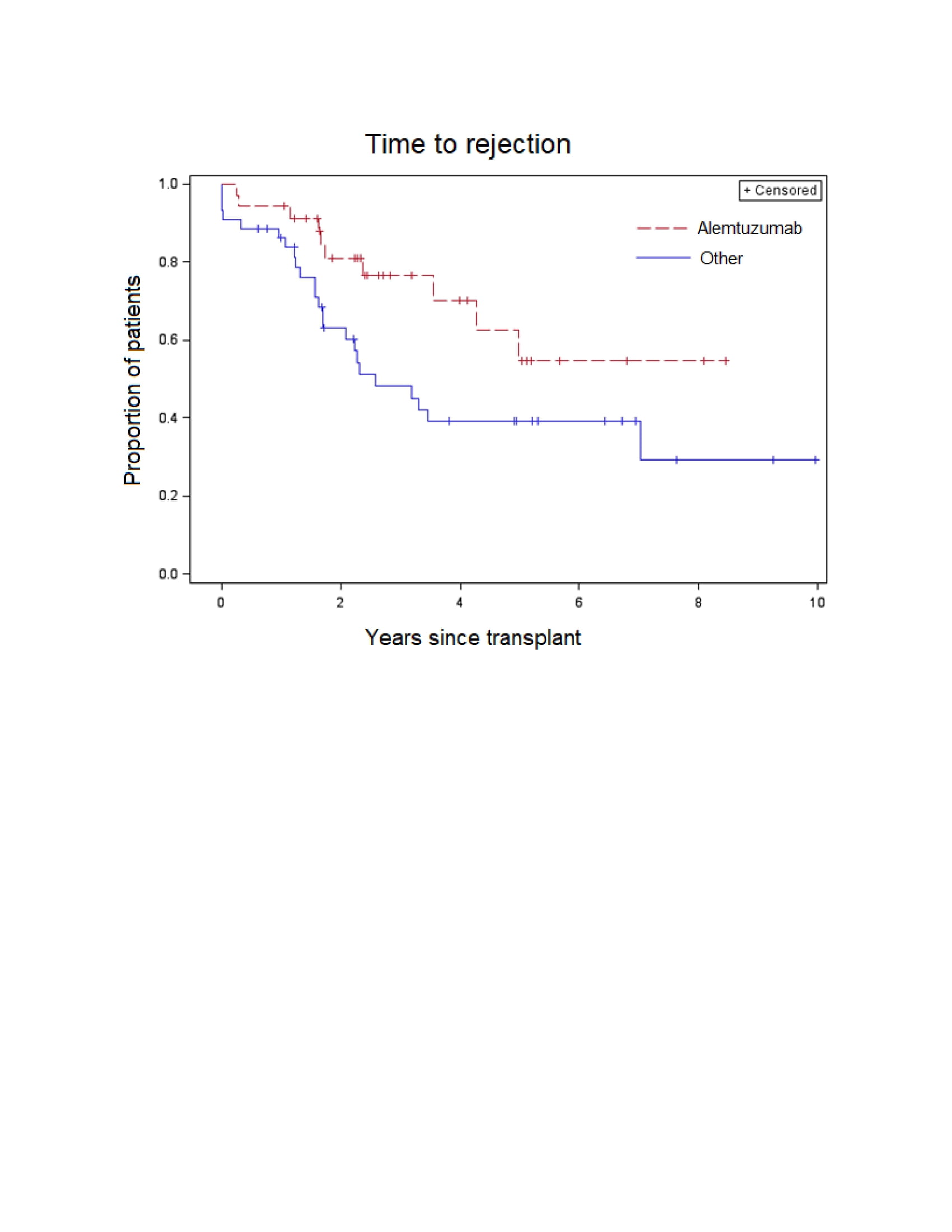
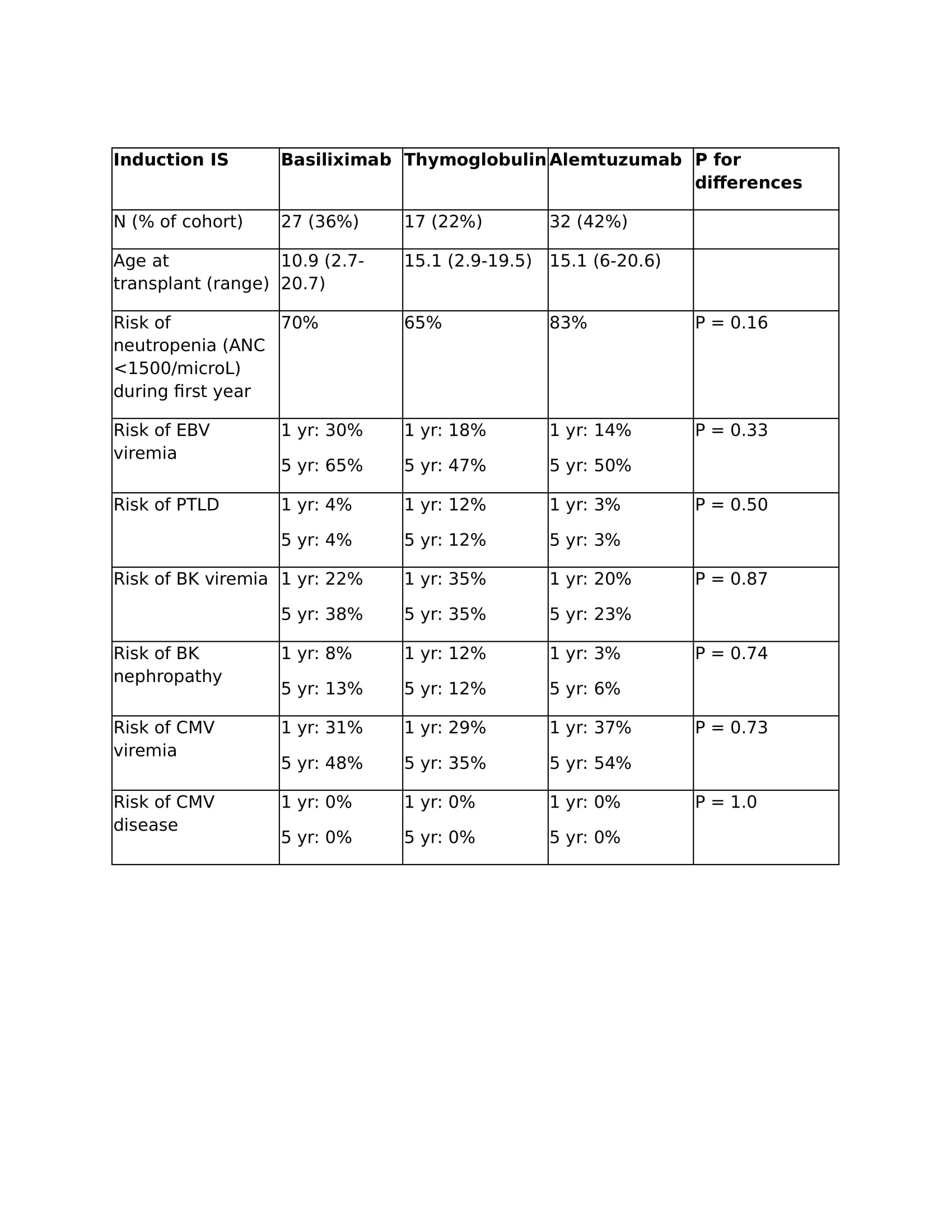
Conclusion: Alemtuzumab reduced the risk of rejection at 1 and 5 years as compared to basiliximab and thymoglobulin in our cohort. There may be a higher risk of neutropenia among alemtuzumab recipients, but the risk of viral infections was similar between groups.
References
1. Engen RM, Bartosh SM. Long-term outcomes of two-dose alemtuzumab induction in pediatric kidney transplantation. Pediatr Transplant. 2024;28(3):e14753. doi:10.1111/petr.14753
2. Jain A, Daoud D, Kees-Folts D, et al. Steroid-free maintenance immunosuppression using alemtuzumab in pediatric kidney transplantation: Long-term longitudinal follow-up. Pediatr Transplant. 2022;26(2):e14173. doi:10.1111/petr.14173
3. Erez DL, Pizzo H, Rodig N, Richardson T, Somers M, on behalf of the NAPRTCS investigators. Outcomes based on induction regimens in pediatric kidney transplantation: a NAPRTCS and PHIS collaborative study. Pediatr Nephrol. 2023;38(10):3455-3464. doi:10.1007/s00467-023-05955-5
[1] immunosuppression
[2] pediatric
[3] kidney
[4] alemtuzumab
[5] outcomes
[6] induction