Pretransplant BK virus immunity in pediatric kidney transplantation: Predictive markers and role of IVIg prophylaxis
Charlotte Duneton1,2, Juliette Rio1, Isabelle Nel3,4, Elodie Cheyssac1, Veronique Baudouin1, Morgane Solis5,6, Samira Fafi Kremer5,6, Guislaine Carcelain3,4, Julien Hogan1,7.
1Pediatric Nephrology, dialysis and transplantation department, Robert Debré Hospital AP-HP, Paris, France; 2INSERM U976, Université Paris Cité, Paris, France; 3Immunology Laboratory, Robert Debré Hospital AP-HP, Paris, France; 4INSERM UMR1141, Université Paris Cité, Paris, France; 5Virology Laboratory, Strasbourg University Hospitals, Strasbourg, France; 6Unité Mixte de Recherche 1109, Institut National de la Santé et de la Recherche Médicale, Strasbourg University, Strasbourg, France; 7Paris Translational Research Center for Organ Transplantation, INSERM, UMR-S970, Université Paris Cité, Paris, France
Introduction: BK virus–associated nephropathy (BKVAN) is a major cause of kidney graft loss, with no specific antiviral therapies available. The current clinical approach relies on minimizing immunosuppression based on viral load monitoring. Nevertheless, this empirical strategy does not consistently yield success, underscoring the importance of identifying pretransplant predictors of BKV replication and developing effective preventive treatments.
Methods: We assessed pretransplant Anti-BKV neutralizing antibody (NAb) titers in 42 pediatric kidney transplant recipients (April 22–24). Patients with BKV Genotype I NAb≥4 log10 IC50 (seropositive) did not receive prophylaxis, while seronegative patients (BKV Genotype I NAb< 4 log10 IC50) underwent preventive IVIg administration (0.3-0.4 g/kg/3 weeks for 6 months). IVIg supplementation in the early months post-transplant is a strategy that has proven effective in adult patients, as IVIg, derived from healthy donors, contains naturally occurring neutralizing antibodies against BK virus, given the widespread prevalence of the virus in the general population. Additionally, analysis of pretransplant anti-BKV cellular responses was conducted using BKV-Elispot assays in 21/42 patients to assess its predictive value.
Results: Among the 42 patients analyzed, BKV viremia occurred in 6/17 (35%) NAb-positive patients, compared to 11/25 (44%) NAb-negative patients. Similarly, BKVAN was diagnosed in 2/17 (11.8%) NAb-positive patients and 3/25 (12%) NAb-negative patients. No statistically significant difference in posttransplant BKV replication or nephropathy was observed between the two groups. However, when comparing patients who developed BKV replication to those who did not, lower pretransplant NAb titers were associated with a higher risk of posttransplant BKV replication, regardless of IVIg supplementation.
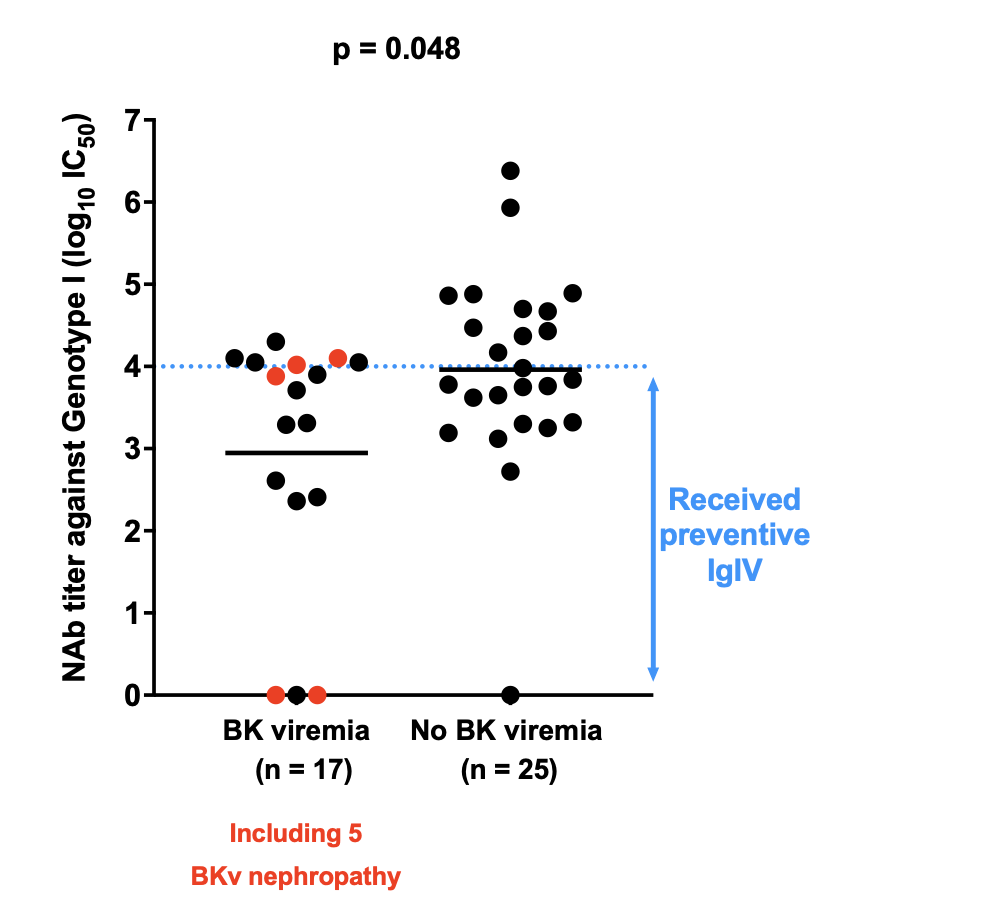
Since all seronegative patients received IVIg supplementation, it is not possible to assess the efficacy of IVIg as a preventive treatment. Therefore, we added an additional analysis by including 50 historical controls who did not receive IVIg, and pretransplant frozen serum from these patients are currently being analyzed to determine their BKV NAb status. 21 BKV Elispot assays were performed on PBMCs collected on the day of transplantation. While the limited sample size prevents us from reaching statistical significance, it is noteworthy that patients who developed posttransplant BKV replication tended to exhibit lower pretransplant cellular responses compared to those who did not experience BKV replication.
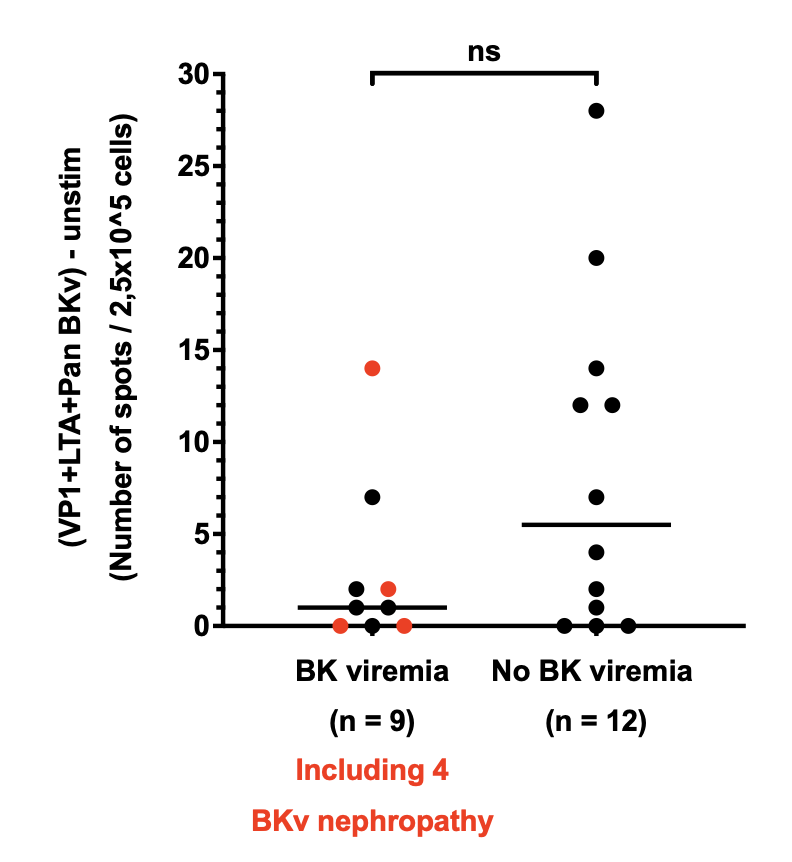
Conclusion: Pretransplant anti-BKV NAb titers appear to be associated with the risk of posttransplant BKV replication in pediatric kidney recipients. The impact of IVIg supplementation in seronegative patients requires further investigation through the ongoing analysis of historical controls. Additionally, anti-BKV cellular response may serve as a promising predictive biomarker.
[1] Kidney transplant
[2] pediatrics
[3] BK virus