
Donor derived cell-free DNA is higher in small pediatric transplant recipients receiving kidneys from obese donors
Julia Steinke1, Lyndsay Harshman2, Kelly Kirshner7, Arundhati Kale3, Erica Winnicki4, Daniel Ranch5, Jayanthi Chandar6, Helen Pizzo7, Arshdeep Kaur2, Paul Hanson8, Ling Chen8, Zuinqui Chen8, Dechu Puliyanda7.
1Pediatric Nephrology, Helen DeVos Children's Hospital, Grand Rapids, MI, United States; 2Pediatric Nephrology, University of Iowa Stead Family Children's Hospital, Iowa City, IA, United States; 3Pediatric Nephrology, University of California, Davis, CA, United States; 4Pediatric Nephrology, University of California, San Francisco, CA, United States; 5Pediatric Nephrology, University of Texas Health Science Center, San Antonio, TX, United States; 6Pediatric Nephrology, University of Miami Miller School of Medicine, Miami, FL, United States; 7Pediatric Nephrology, Cedars Sinai Guerin Children's Hospital, Los Angeles, CA, United States; 8Biostatistics Core, CareDx, Brisbane, CA, United States
Purpose: Donor derived cell free DNA (dd-cfDNA) has increasingly been used as a non-invasive surrogate marker of kidney transplant (KT) injury. The interpretation in pediatric patients (pts) using cutoff values derived from adult data has been brought into question especially with donor:recipient size mismatch. In our cohort of pediatric KT recipients, we aimed to define cutoff values in dd-cfDNA below which rejection or BK nephropathy is unlikely, and determined if there were variations in dd-cfDNA in recipients based on donor weight and BMI.
Methods: Seven pediatric KT centers in the USA provided retrospective data. Data from clinically stable pts <18 years (defined as no biopsy proven rejection or BK viremia/nephropathy), with at least 2 dd-cfDNA samples per year, were included for this analysis. Recipients were categorized by weight (wt) (<20 kg, 20-50 kg, and >50 kg) at the time of dd-cfDNA collection. Donor body mass index (BMI) was categorized by BMI (underwt ≤18.5, normal 18.6-24.9, overwt 25-29.9, obese ≥30). Donor BMI and recipient wt categories were compared using linear mixed models for analysis. Values were expressed as mean +/- SD, median and interquartile range.
Results: Of 265 pts screened, 178 clinically stable pts were included for analysis. There was no difference in mean dd-cfDNA values based on recipient wt (0.51 +/- 0.44 <20 kg, 0.65 +/- 1.28 20-50 kg, 0.49 +/- 0.7 >50 kg). Upon comparison of donor BMI to recipient wt, mean dd-cfDNA in pediatric pts with low BMI donors were 1.6 times higher compared to normal BMI donors (n 42, p 0.036). Median values of dd-cfDNA did not differ based on donor BMI (0.28 (0.2-0.81), 0.29 (0.18-0.51), 0.26 (0.18-0.49), 0.27(0.18-0.46)). Among overwt and obese donors, dd-cfDNA was 1.3 times higher in <20 kg recipients (n 74, p 0.044). No association was found between dd-cfDNA and donor:recipient BMI.
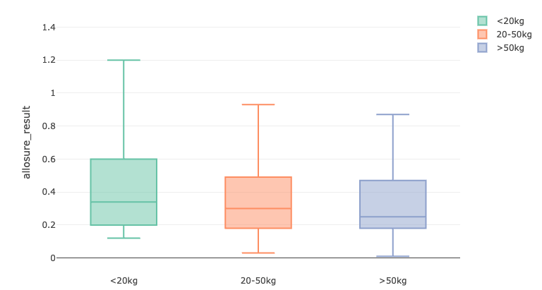
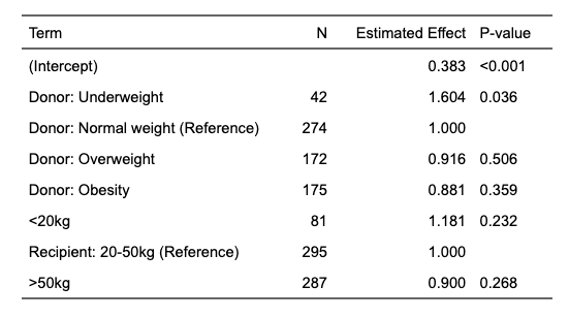
Conclusions: This multicenter pediatric study demonstrates that dd-cfDNA values in stable pediatric patients do not vary based on recipient weight or the donor-to-recipient BMI ratio, with average values remaining below the 1% cutoff. However, higher donor BMI was associated with a 1.3-fold increase in dd-cfDNA levels in small recipients. While the average dd-cfDNA results in recipients with low BMI donors were 60% higher and showed the greatest variability, median values remained comparable across donor BMI categories.
[1] donor derived cell free DNA
[2] kidney transplant