Maria Teresa Ludovica Viganoni, Italy
Resident
Pediatric Nephrology- Dialysis and Transplantation Unit,
Fondazione IRCCS Ca' Granda- Ospedale Maggiore Policlinico
Cytomegalovirus infection in first year after pediatric renal transplantation: a ten year single center experience
Maria Teresa Ludovica Viganoni1,2, Sara Testa2, Marco Cugliari1,2, Silvia Consolo2, Fabio Paglialonga2, Martina Carucci4, Piero Palma3, Giovanni Montini1,2.
1Department of Clinical Science and Community Health, Milan, Italy., University of Milan, Milan, Italy; 2Pediatric Nephrology- Dialysis and Transplantation Unit, Fondazione IRCCS Ca' Granda- Ospedale Maggiore Policlinico, Milan, Italy; 3Department of Woman- Child and General and Specialized Surgery S, University of Campania "Luigi Vanvitelli", Naples, Italy; 4Department of medicine and surgery "Scuola Medica Salernitana", University of Salerno, Salerno, Italy
Aims: Cytomegalovirus (CMV) infection is a common complication post pediatric kidney transplant (KT). Valganciclovir (VCG) is indicated for prophylaxis and treatment. This study aims to define CMV infection epidemiology during the first year post-KT and to evaluate the influence of VCG on kidney function.
Methods: We conducted a retrospective study including pediatric patients (<21 years) who received KT between January 2014 - February 2024, with a ≥ 12 months follow-up.
The immunosuppression protocol consisted of Basiliximab or Thymoglobuline for induction, followed by Calcineurin inhibitors, Mycophenolate mofetil, Azathioprine or Everolimus, and Prednisone for maintenance.
Patients were risk-stratified based on pre-transplant CMV serostatus: high-risk (donor+/recipient-), medium-high risk (donor+/recipient+), medium-low risk (donor-/recipient+) and low-risk (donor-/recipient-).
A 3-month VCG-prophylaxis was administered to the high-risk group and to those who received ATG induction. CMV-DNAemia was defined by a CMV polymerase chain reaction test result >250 copies/mL in blood. VCG pre-emptive treatment was initiated if CMV DNAemia exceeded 500 copies/mL. Follow-up data were collected at baseline and months 3-6-9-12 after KT.
Results: Of the 137 transplanted patients, 104 met the inclusion criteria, while 33 were excluded due to loss to follow-up (27), death (2), acute rejection (1), primary disease relapse (1), renal vein thrombosis (1) and infection (1).
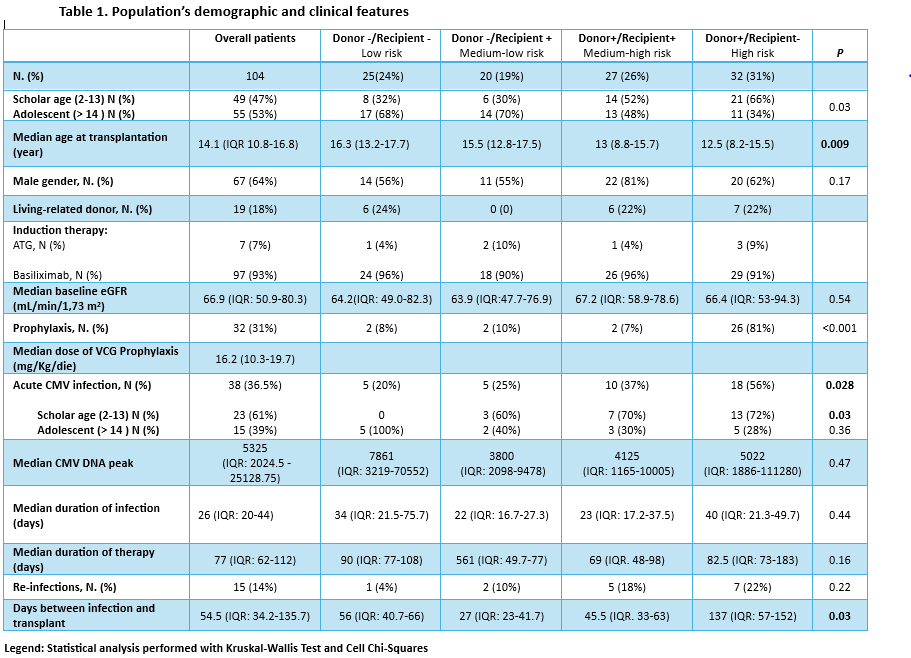
Table 1 summarizes features of our cohort.
VCG prophylaxis was administered to 32/104 patients (31%) with a median dose of 17 mg/kg/die (IQR:10.4-19.7). CMV DNAemia was detected in 38/104 (36.5%) overall patients: 47% in high-risk, 27% in medium-high risk, 13% in medium-low risk, and 13% in low-risk group respectively. The incidence was significantly different between risk groups (p=0.03). Splitting the groups by age we noted that the difference between risk groups was more evident in the scholar-age patient group. In high-risk group, infection occurred at a median time of 53 days (IQR:26.5-64.5) after prophylaxis ended, with a median infection duration of 40 days (IQR:21.3-49.7), longer but not statistically significant compared to other groups. Within the medium-risk group, donor+/recipient+ showed higher infection rates compared to donor-/recipient+ (p=0.37), however not statistically significant.
VCG treatment was administered with a median dose of 30 mg/kg/die (IQR 21.2-39.1). No major adverse events were noted. VCG-treated and non-VCG-treated patients had similar median ΔGFR (3-12 month): 8.55 vs. 8.6 mL/min/1.73 m² respectively.
Conclusions: Paediatric KT recipients are at high risk for CMV-DNAemia despite VCG prophylaxis, which does not affect renal function. Given the early post-prophylaxis infection onset in the high-risk group, extended prophylaxis may be beneficial. Moreover, prophylaxis may be also indicated for the medium-high risk group, particularly for younger patients.
Lectures by Maria Teresa Ludovica Viganoni
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sat-20 17:40 - 18:40 |
Infectious Disease/ PTLD and Malignancy Posters - from P2.63 to P2.73 | Cytomegalovirus infection in first year after pediatric renal transplantation: a ten year single center experience | MOA 10 (Exhibit Area) |
