Sirolimus monotherapy for maintenance of immunosuppression after pediatric liver transplant
Sanjay Rao1, Reya Abraham1, Zameer M M1, Vinay Chandrashekar1, Sahana Shankar2, Rakesh M1, Ashley D'Cruz1.
1Pediatric Surgery and Transplantation, Narayana Health Hospitals, Bangalore, India; 2Pediatric GI, Hepatology and Nutrition, Narayana Health Hospitals, Bangalore, India
Introduction: Tacrolimus forms the backbone of immunosuppression after pediatric liver transplantation. However, calcineurin inhibitors such as tacrolimus are associated with significant side-effects. M-TOR inhibitors such as Sirolimus are increasingly being used in children- reduced renal side effects, some protection against EBV and PTLD are some of the potential benefits. We describe our experience with sirolimus as a single agent for maintenance immunosuppression in children after liver transplant.
Methods: A retrospective review of records was carried out and children who were switched from CNI to sirolimus were identified. Demographic information, indications for the switch, timelines and outcomes were studied.
Results: Eleven children in our cohort of 83 pediatric liver transplant recipients were switched to single agent sirolimus. There were 6 boys. At transplant, their mean age was 19.6months (range 7-60 months) and mean weight was 8.6kg (range 5.75-13kg). All children received living donor left lateral segment grafts from one of their parents. Initial immunosuppression was dual drug - steroid and tacrolimus. Steroid was weaned off by 90 days post-transplant and single drug tacrolimus was continued. Persistently elevated levels of serum EBV titers, renal preservation and PTLD were the indications for change. The details are listed in Table 1. The mean duration from transplant to switching to sirolimus was 23 months (range 8 to 75 months). The mean follow-up while on sirolimus was 78.7 months (20 to 172months) Four of these children had acute cellular rejection while on tacrolimus, these were treated with steroids. However, after switching to sirolimus, there had been no further rejections. The liver function tests were normal, and liver appeared normal on ultrasound-protocol biopsies were not performed. Lipid profiles remain normal.
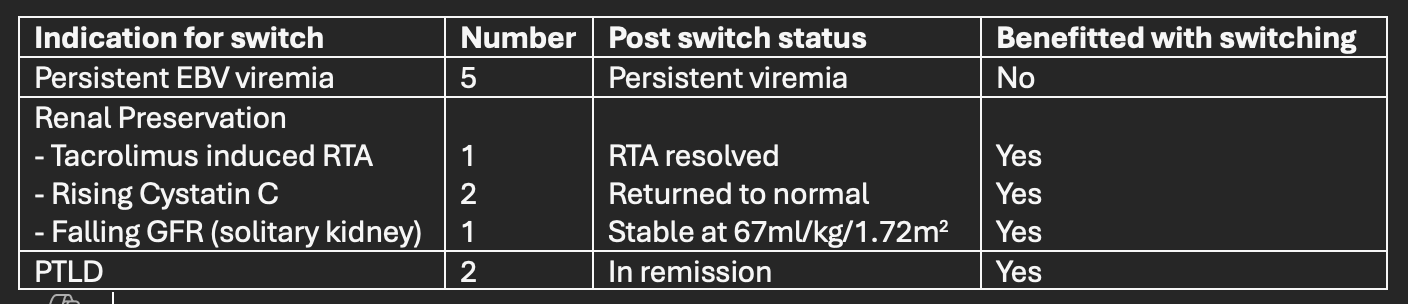
Conclusions: Sirolimus as a single immunosuppressive agent is effective in preventing acute rejections and is well tolerated – in the medium term. Switching to sirolimus effectively reversed side effects of tacrolimus and maintained GFR. Recipients with PTLD remained in remission. However, despite its known anti-EBV effects, sirolimus had no effect in reducing levels of EBV titers. While the study is limited by small sample size, the follow up is on average 6 years after switching.
Lectures by Sanjay Rao
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sat-20 17:40 - 18:40 |
Liver/Intestine Posters - from P2.35 to P2.56 | Sirolimus monotherapy for maintenance of immunosuppression after pediatric liver transplant | MOA 10 (Exhibit Area) |
