Center for Postgraduate Education and Training
National Center for Child Health and Development
Impact of pre-transplant anti-CD20 antibody administration on post-transplant EBV infection after pediatric liver transplantation
Koushin Kuroki1,2,3, Kosuke Kirino4, Naoya Tonegawa1, Chikara Ogimi2, Isao Miyairi3, Hajime Uchida5, Akinari Fukuda5, Seisuke Sakamoto5, Mureo Kasahara5, Masaki Yamada2.
1Center for Postgraduate Education and Training, National Center for Child Health and Development, Tokyo, Japan; 2Division of Infectious Diseases, Department of Medical Subspecialties, National Center for Child Health and Development, Tokyo, Japan; 3Department of Pediatrics, Hamamatsu University School of Medicine, Shizuoka, Japan; 4Department of Data Science, Clinical Research Center, National Center for Child Health and Development, Tokyo, Japan; 5Organ Transplantation Center, National Center for Child Health and Development, Tokyo, Japan
Introduction: EB virus (EBV) is a type of herpesvirus that primarily infects B cells; EBV is a major cause of post-transplant lymphoproliferative disorders (PTLD). To prevent the development of PTLD following post-transplant EBV infection, anti-CD20 antibody (rituximab) has been administered in hematopoietic cell transplant recipients. On the other hand, few studies have shown the positive effect of rituximab in preventing EBV infection and PTLD in solid organ transplant recipients who require life-long immunosuppressive therapy. Of note, rituximab has also been used before pursuing ABO-incompatible transplantation to decrease anti-ABO antibodies. However, few studies have investigated the potential impact on the risk of EBV infection in these patients. Therefore, this study aimed to assess the impact of rituximab administration on EBV infection in pediatric liver transplant recipients.
Methods: Children under 18 years of age who underwent their first isolated liver transplantation at our institution from July 2014 to December 2022 were included. We analyzed the occurrence of EBV infection in the rituximab-treated and non-rituximab-treated groups, using chi-square and Kaplan-Meier curve analysis to compare the two groups. A p-value less than 0.05 was considered statistically significant.
Results: Four hundred forty-six pediatric liver transplant recipients met the inclusion criteria for this study. Of these, 14 were treated with rituximab and 432 were not. Kaplan-Meier analysis demonstrated a significantly lower cumulative EBV infection rate at 2 years post-transplant in the rituximab group (74.1% vs. 35.7%) (P = 0.010).
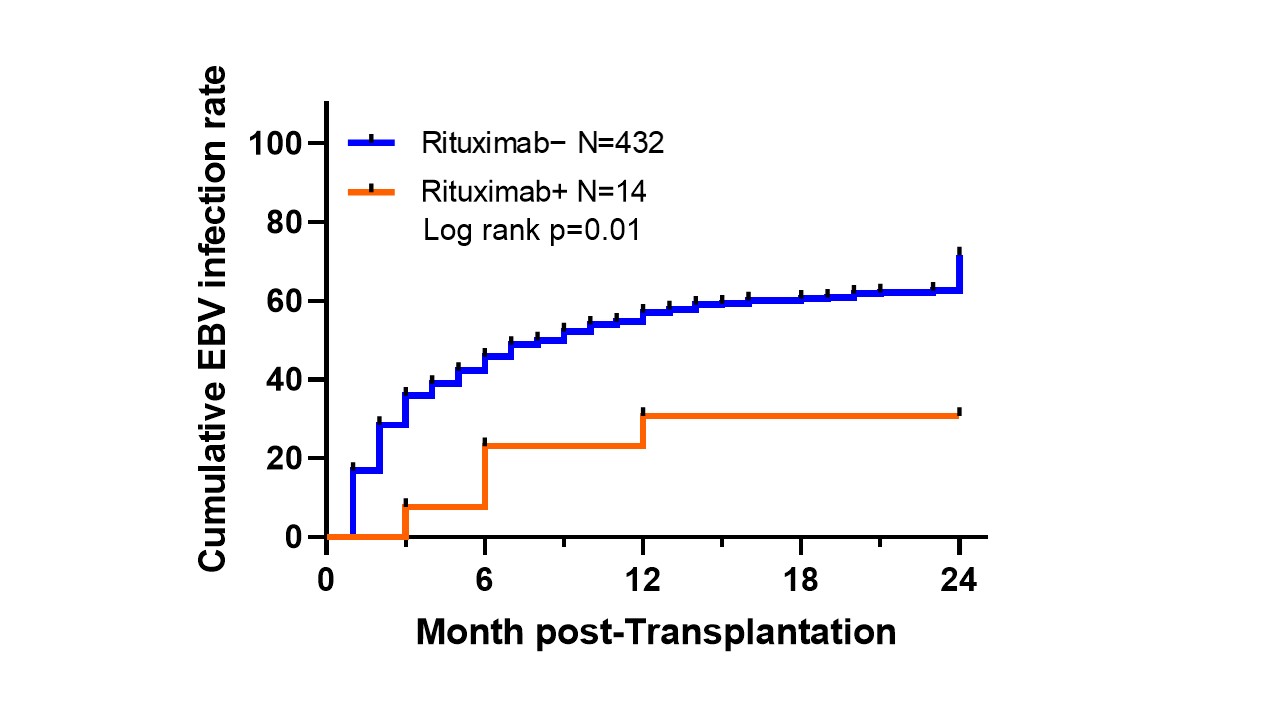
Conclusion: Pediatric liver transplant recipients treated with rituximab due to ABO incompatibility have reduced EBV infection late in the early post-transplant period compared to controls. These results suggest that rituximab treatment may effectively prevent PTLD, but further studies are needed, including analysis of more cases with adjusted patient backgrounds.
Lectures by Koushin Kuroki
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sat-20 10:00 - 11:00 |
Infections and vaccines | Impact of pre-transplant anti-CD20 antibody administration on post-transplant EBV infection after pediatric liver transplantation | MOA 5 |
