Pediatric Nephrologist
Department of Pediatric Nephrology
Hospital de Clínicas, Faculty of Medical Sciences, National University of Asunción.
Lymphoproliferative disease in kidney transplanted children: A single center experience from Argentina
Marta L Monteverde1, Gabriela Gutièrrez1, Juan Ibáñez 1, Claudia Sarkis 2, Diana Di Pinto1, Veronica Solernou3, Daniela Borgnia4, Catrian Sotelo5.
1Renal transplant section, Nephrology Unit, Hospital JP Garrahan, Ciudad de Buenos Aires, Argentina; 2Infectious Diseases Unit, Hospital JP Garrahan, Ciudad de Buenos Aires, Argentina; 3Pathology Unit, Hospital JP Garrahan, Ciudad de Buenos Aires, Argentina; 4Virology Section, Hospital JP Garrahan, Ciudad de Buenos Aires, Argentina; 5Oncology Unit, Hospital JP Garrahan, Ciudad de Buenos Aires, Argentina
Introduction: In children with transplants, lymphoproliferative disorders are cause of mortality and morbidity. Most of them are associated with Ebstein Barr virus. Aim: 1) To describe, in a cohort of kidney transplant (KT) patients, cumulative incidence, incidence rate, time to onset post-KT, and clinical and histopathologic features of patients (pt) with PTLD. 2) To compare pt and graft survival with and without PTLD. 3) In EBV seronegative pt (viral capsid antigen-VCA-IgG neg before KT), analyze association between peri KT administration of rituximab, primary EBV infection, and development of PTLD
Methods: Retrospective study, including 1072 consecutive KT pt at a tertiary-care center, (Aug 1988-Dec 2023). PTLD was diagnosed according to WHO 2017 criteria. Early PTLD was defined as <1 y post KT, and late PTLD > 1 y post KT
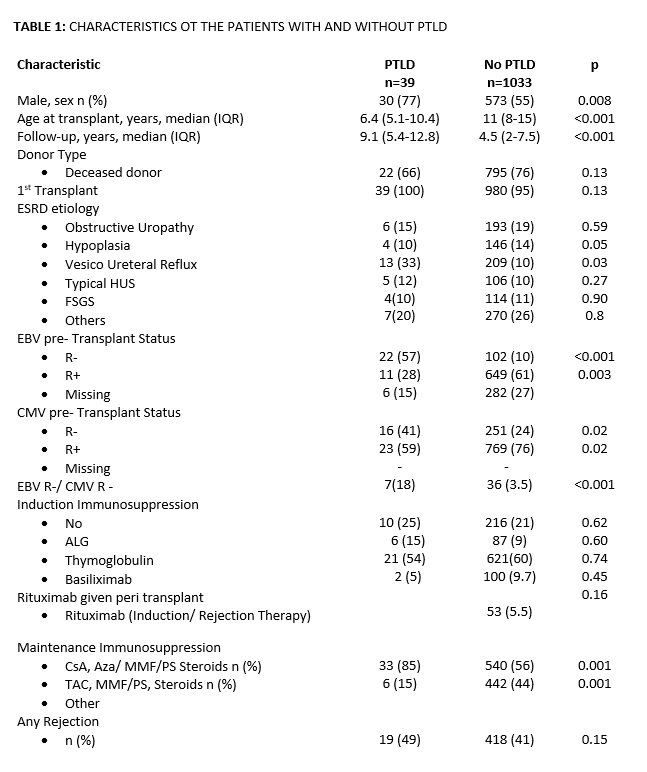
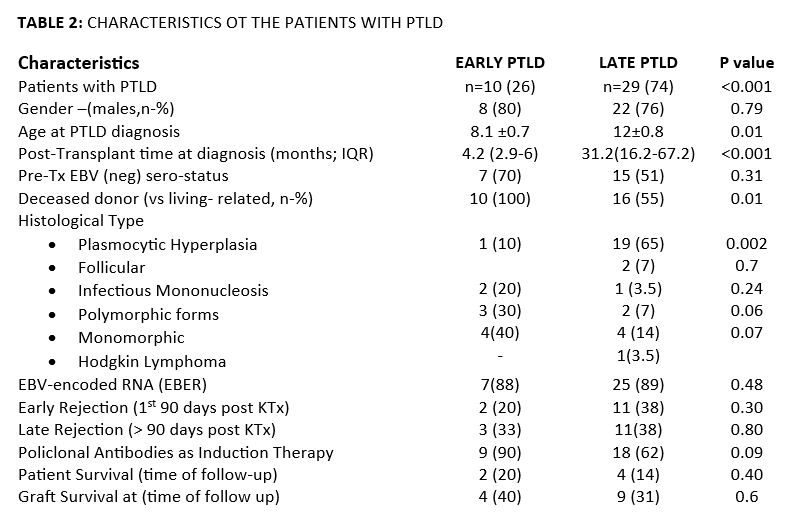
Results: Since 1988, PTLD was diagnosed in 39 pt. PTLD incidence rate was 4.5 per 100 persons-years (py). Before 2012, when viral load monitoring was not available, it was 5 per py; after 2012, 3 per py. PTLD risk was 3.6%; 5.4 % before 2012, and from 2012 to now 1.13% (p<.001). With PTLD were mostly male, younger, with a longer time of follow-up. EBV naive pt, compared to those positive, had a mean 5.8 times higher risk (CI95%:4-8) of PTLD, those CMV neg (CMV IgG neg) 2.1 higher risk (CI95%:1.1-3.9), and those both EBV and CMV neg (n=7), 5.3 times higher risk (CI95%:2.5-11). Histologically, 25 had early lesions, five polymorphic (PM), eight monomorphic (MM), and one pt Hodgkin’s disease. Median time from KT to PTLD diagnosis was 25.4 m (IQR:12.4-63.7), and median pts’ age 10.3 y (IQR:6.9-14.5). Ten pt, deceased donor recipients of younger age at diagnosis (p<.01) developed early PTLD (p<.01), In them, MM and PM were the most frequent forms (70%; p=.004). In late PTLD, plasmocytic hyperplasia was most common (p=.002). All pt with PM PTLD were EBV-naïve KT (p=.03), and 6/8 MM. Considering them as a group, EBV-neg pt with PTLD (n=11) had a mean 2-fold higher risk (95% CI:1.2-3) of having a MM o PM form versus others; if they received polyclonal antibodies (n=15), the risk increased (HR: 3 CI95%:1.3-6.6). Pt survival at 1-, 3-, and 5-after KT were 97%, 92%, and 89%, and 99%, 97.6%, and 96.4%, in those with vs without PTLD, respectively (p=.011). Six children died at 2.6 m (IQR:0.7-8.3) after diagnosis. Risk of death in patients with PM or MM PTLD was 2.4 times higher (95%CI: 1.1-5.4). The 1-, 3-, and 5-year graft survival with and without PTLD were 92.3%, 89.7%, and 87%, and 93%, 86%, and 81%, respectively (p=.36). Twelve pt EBV naive received Rituximab (one dose, 375 mg/m2) in peri KT. In their follow-up, neither had increased EBV loads or developed PTLD
Conclusion: In this cohort, incidence of PTLD decreased. EBV naive pt had higher risk of PTLD. In those with PTLD patient survival was worse. Peri KT Rituximab given to EBV-neg recipients was associated with absence of PTLD during follow-up
References:
[1] PTLD
[2] EBV VIRUS
[3] Lymphoproliferative
Lectures by Gabriela Gutièrrez
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sat-20 10:00 - 11:00 |
Infections and vaccines | Lymphoproliferative Disease in kidney transplanted children: A single center experience from Argentina | MOA 5 |
|
Sat-20 11:05 - 12:05 |
Tips for early career professionals | Short presentations from the 1st cycle of IPTA mentoring | MOA 6 |
