CareDx
Donor derived cell-free DNA is elevated during rejection across all ages in pediatric heart transplant recipients
Brian Feingold1, Deepa Mokshagundam2, Gary Beasley3, Melanie Everitt4, Kris Oreschak5, Sijia Wang5, Jeremy Kobulnik5, Ling Shen5, Defne Magnetta6.
1UPMC Children's Hospital of Pittsburgh, Pittsburgh, PA, United States; 2Washington University in St Louis, St. Louis, MO, United States; 3University of Iowa Stead Family Children's Hospital, Iowa City, IA, United States; 4Children's Hospital Colorado, Aurora, CO, United States; 5CareDx, Brisbane, CA, United States; 6Lurie Children's Hospital of Chicago, Chicago, IL, United States
Introduction: Acute rejection (AR) is a major contributor to graft failure following pediatric heart transplant (HTx), but timely detection and treatment of rejection episodes remain a significant clinical challenge. Initial studies suggest donor-derived cell-free DNA (dd-cfDNA) can sufficiently discriminate AR from no rejection (NR) after pediatric HTx; however, most participants in these studies were older pediatric patients. To date, no studies have assessed whether dd-cfDNA can successfully identify AR in different pediatric HTx age ranges. The goal of this analysis was to determine whether dd-cfDNA levels are elevated during AR in different age groups following pediatric HTx.
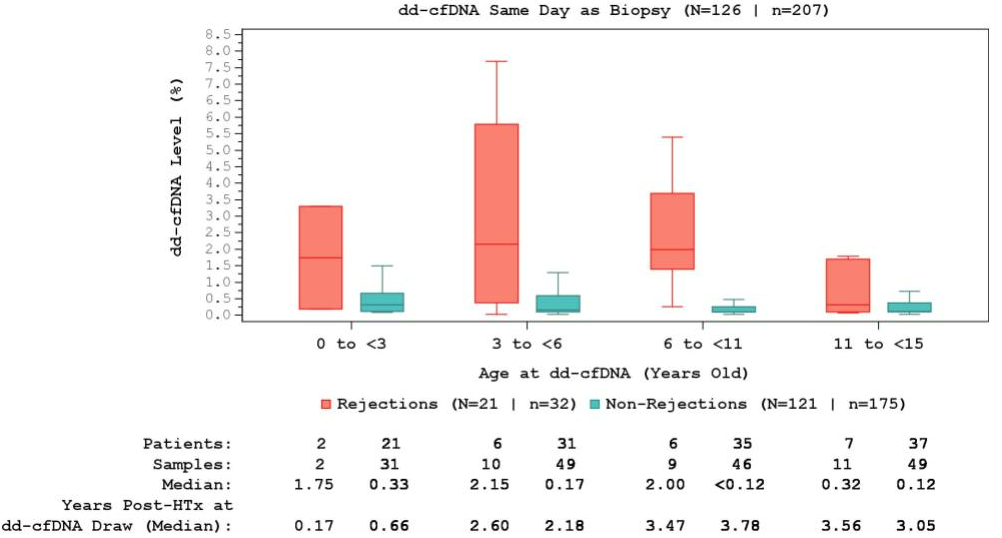
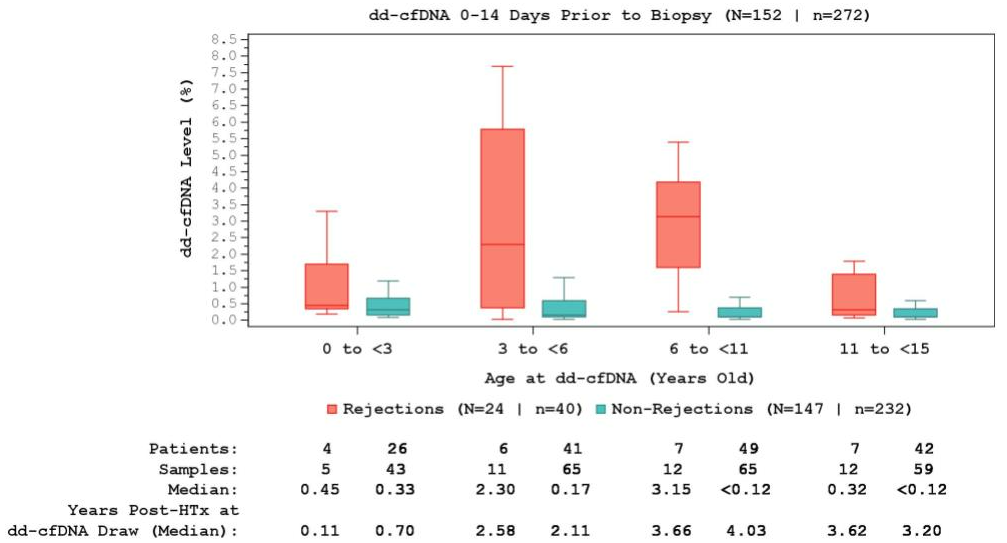
Methods: This 4-center analysis included single-organ pediatric HT recipients <15 years old at the time of dd-cfDNA testing with ≥1 paired dd-cfDNA and endomyocardial biopsy (EMB) performed on the same day. A second analysis using a dd-cfDNA window of 0-14 days before EMB was also performed. AR was defined as biopsy-proven acute cellular rejection (ACR) grade ≥2R and/or antibody mediated rejection (AMR) grade ≥pAMR1. NR was defined as an ACR grade <2R and pAMR0 on EMB. dd-cfDNA levels were assigned to one of four groups based on the patient’s age at time of blood draw: 0- to <3-years-old, 3- to <6-years-old, 6- to <11-years-old, 11- to <15-years-old. A mixed model analysis was conducted to evaluate the interaction between rejection status and age at dd-cfDNA draw on dd-cfDNA test results.
Results: This analysis included 126 HT recipients <15 years old. Amongst patients with complete demographic data, the cohort was 61% white and 50% male, with a median age at HT of 3 (IQR 1-9) years. There were 207 dd-cfDNA paired on the same day as EMB, of which 175 (85%) across 121 patients showed NR and 32 (15%) from 21 patients had AR. AMR-alone accounted for 81% (n=26) of AR, with ACR-alone representing 16% (n=5) of AR cases. One (3%) EMB had both ACR and AMR. The median time post-HT of dd-cfDNA for AR and NR were 3.2 and 1.8 years, respectively. Median same day dd-cfDNA levels were significantly higher during AR than NR in all patients (1.55% vs 0.16%, p<0.001). dd-cfDNA levels were numerically higher during AR when compared to NR samples for all age groups when including dd-cfDNA and EMBs performed on the same day (Figure 1) or dd-cfDNA levels drawn 0-14 days prior to EMB (Figure 2). There was no significant interaction between dd-cfDNA levels in NR vs AR and age (p=0.30). dd-cfDNA levels associated with NR were higher in patients 0 to<3-years-old compared to other age groups.
Conclusions: dd-cfDNA was consistently elevated in AR compared to NR in all pediatric age groups following HTx. Further research is needed to understand differences in dd-cfDNA levels between pediatric patients and levels seen in adult studies.
Lectures by Kris Oreschak
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sat-20 10:00 - 11:00 |
Special topics in transplantation | Donor derived cell-free DNA is elevated during rejection across all ages in pediatric heart transplant recipients | MOA 3 |
