Rady Children's Pediatric Nephrology
Assessment of DSA by serial titration associates with acute rejection severity in pediatric kidney transplant recipients
Clarkson Crane1, Janara Mehrabli1, Elizabeth Ingulli1, Gerald P Morris1.
1UC San Diego & Rady Children's Hospital, San Diego, CA, United States
Background: Multiplexed flow cytometry-based single antigen bead (SAB) assays are the standard for detection of anti-HLA alloantibodies. The presence of donor-specific antibodies (DSA) is diagnostic in antibody-mediated rejection (AMR) and higher levels of DSA are associated with risk of AMR. The output of the SAB assay, Mean Fluorescence Intensity (MFI), is correlated with antibody concentration and higher MFI with AMR risk. However, limitations of MFI including non-linearity, saturation, reagent variability, and potential for assay inhibition limit assessment of DSA levels and risk assessment. We tested the hypothesis that DSA quantification by titration is a more informative measure of antibody quantity and is associated with biopsy-proven acute rejection in a cohort of pediatric kidney transplant recipients.
Methods: We performed SAB titration in 8 serum samples from pediatric kidney transplant recipients with known DSA at time of for-cause kidney biopsy. SAB testing was performed using LABScreen Single Antigen Class I and II reagents on a FLEXMAP 3D analyzer with each sample tested by serial dilution (neat, 1:4, 1:10, 1:64). DSA >1000 MFI at any dilution were included for analysis.
Results: There were 10 DSA detected from 8 subjects. Biopsy results included one AMR, five mixed AMR and T cell mediated rejection (TCMR), and three without rejection. Serum dilution demonstrated non-proportional decreases in MFI, highlighting the nonlinear relationship between MFI and DSA concentration (Fig 1). MFI for undiluted serum underrepresented level of DSA in patients with AMR, as these samples showed persistent DSA at 1:10 and 1:64 dilutions, while DSA were not present at these dilutions in non-AMR samples. In those with any AMR, the initial 4-fold DSA dilution resulted in an average 42% increase in MFI (vs 27% for no rejection), followed by 45% and 37% decrease with subsequent dilutions.
Serial titration demonstrated greater sensitivity and a stronger relationship with more severe rejection phenotypes at higher dilutions (Table 1). There were strong correlations between MFI at 1:4 and 1:10 dilutions and Banff interstitial inflammation (i) scores, r = 0.85 (p = 0.01) and r = 0.94 (p = 0.05), respectively. Additionally, there was trend toward an association with MFI at 1:10 dilution and microvascular inflammation on biopsy (Banff g + ptc), r = 0.8 (p = 0.33). Use of 3000 MFI at 1:10 dilution was an optimal cutoff, as this identified all cases of AMR without false positives.
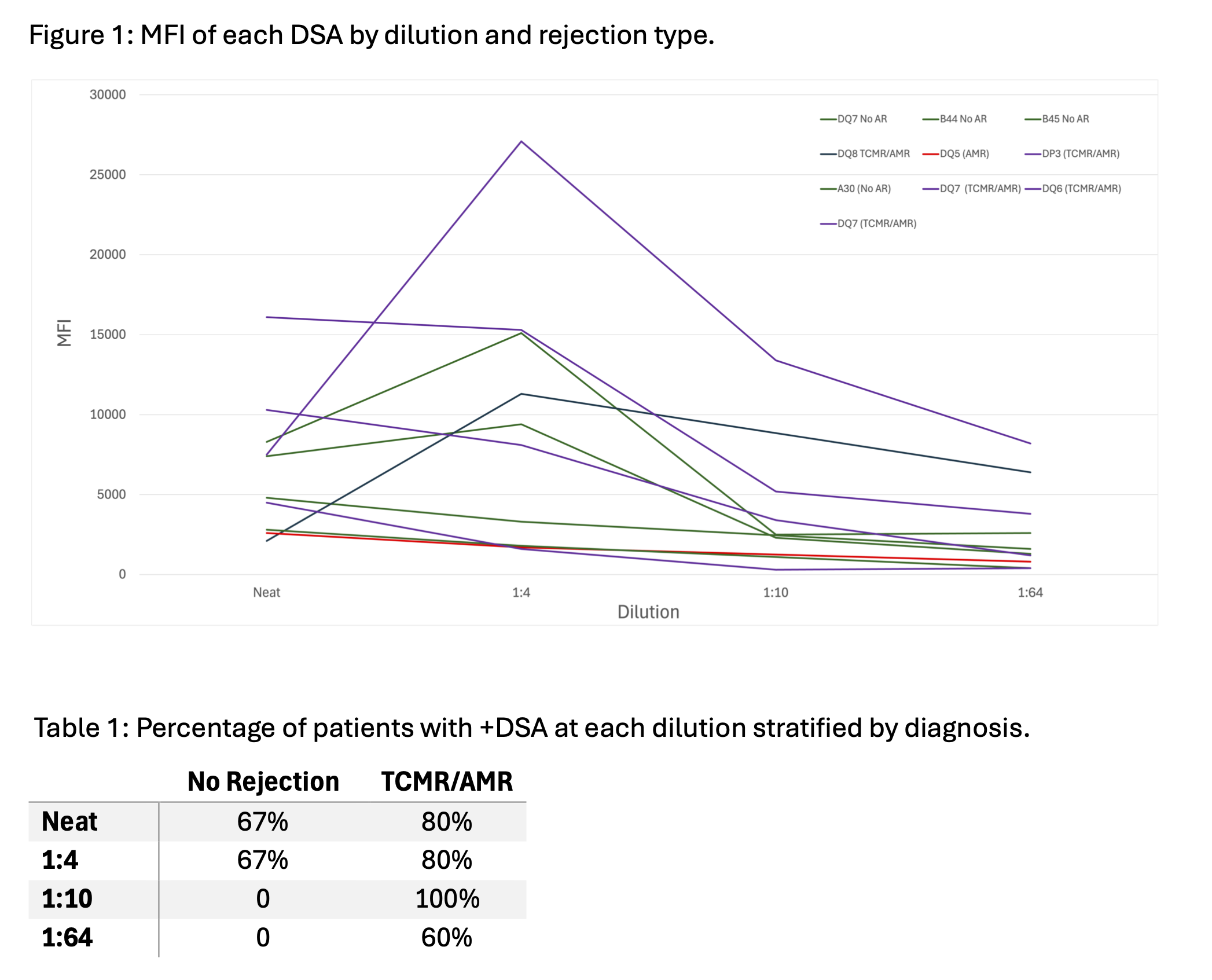
Conclusions: Our results highlight the limitation of using MFI for evaluation of DSA and indicate serum dilution and titration by SAB may provide a more accurate measure of DSA concentration. DSA present at 10-fold dilution correlates with presence and severity of rejection. These results have important clinical implications, particularly when making decisions about the urgency of biopsy or treatment initiation. Future work aims to evaluate reduction in antibody concentration with clinical response to rejection treatment.
We would like to acknowledge Layla Myers (UC San Diego Center for Transplantation) and Joseph Abraha (UC San Diego Immunogenetics and Transplantation Laboratory) for assistance with equipment and experiments..
References:
[1] Kidney
[2] Histocompatibility
