head and Associate Professor
Department of Pediatric Nephrology
Smt. GR Doshi and Smt. KM Mehta Institute of Kidney Diseases and Research Center, Gujarat university of Transplantation Sciences
The induction conundrum: To study the impact of induction immunosuppression on the incidence of rejections and infections, graft and patient survival in pediatric kidney transplant recipients retrospectively
Nayan Chaudhari1, Kinnari Vala1, Shahenaz Kapadia1, Anshuman Saha1, Disha Bhatt1, Suman Choudhary1, Pranjal Modi1.
1Department of Pediatric Nephrology, IKDRC, Ahmedabad, India
Introduction: Induction immunosuppression is critical for kidney transplant outcomes, yet the optimal regimen in pediatric recipients(pKTRs) remains uncertain, particularly in infection-prone regions. This study represents the first and largest Indian pediatric cohort evaluating induction strategies, aiming to balance rejection risk with infection burden. Identifying an effective, region-specific approach is essential to improving transplant outcomes in resource-limited settings. Methodology: This single-center retrospective cohort study analyzed 94 pKTRs (September 2016–July 2024). Patients were stratified by induction immunosuppression: low-dose ATG (L-ATG, <2 mg/kg), basiliximab, ATG-F, high-dose ATG (H-ATG, >2 mg/kg), and rituximab (RTX). Patients who underwent desensitization were excluded. Viral surveillance for EBV, BKV, and CMV was conducted at 1, 3, 6, and 12 months, with protocol biopsies at 6, 12 months, and annually thereafter. Data on demographics, immunological risk, infections, and rejection episodes were collected. Outcomes assessed included graft function, survival, rejection, infection-related hospitalizations, and mortality at 3 months, 6 months, 1 year, and 3 years post-transplant.
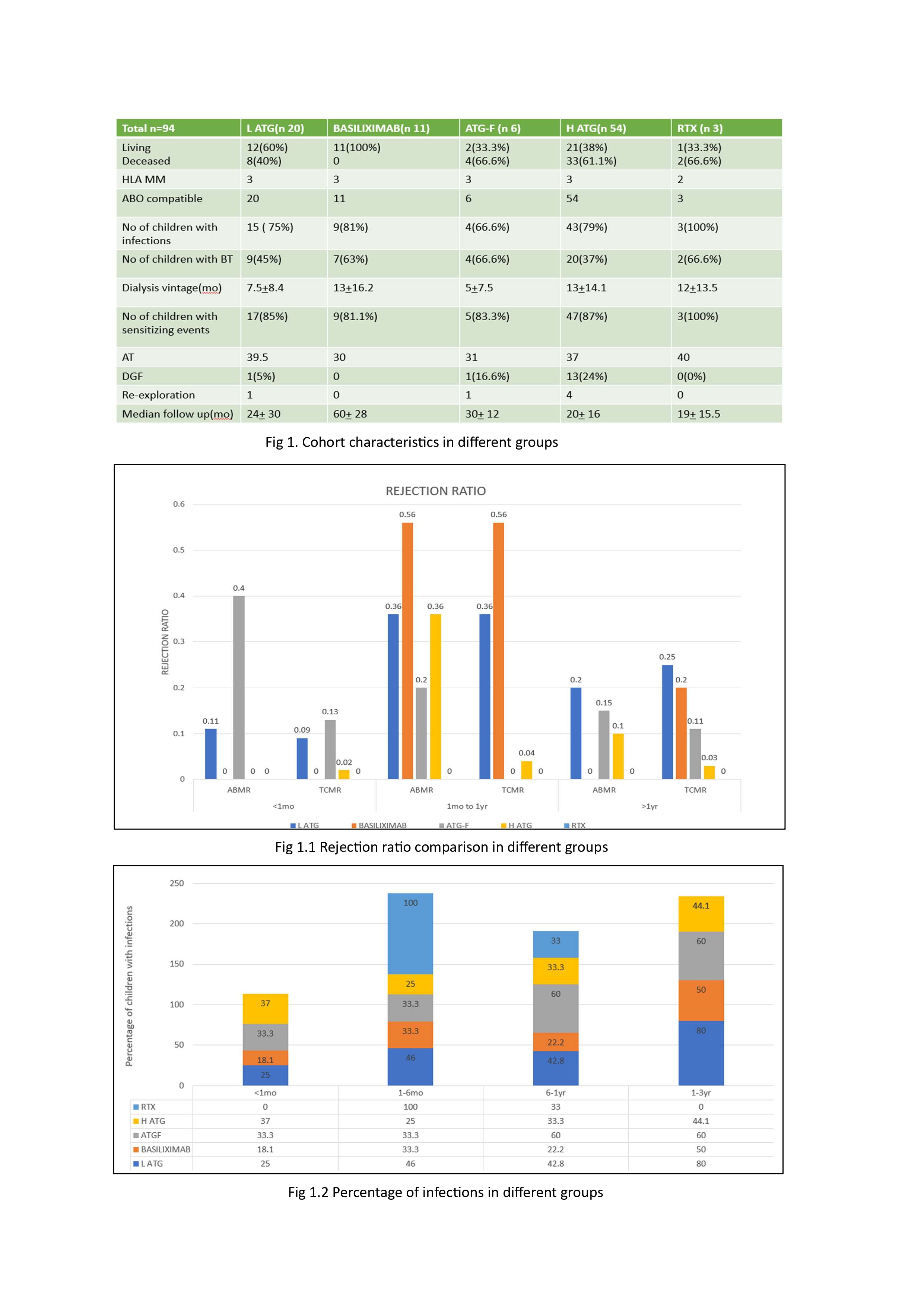
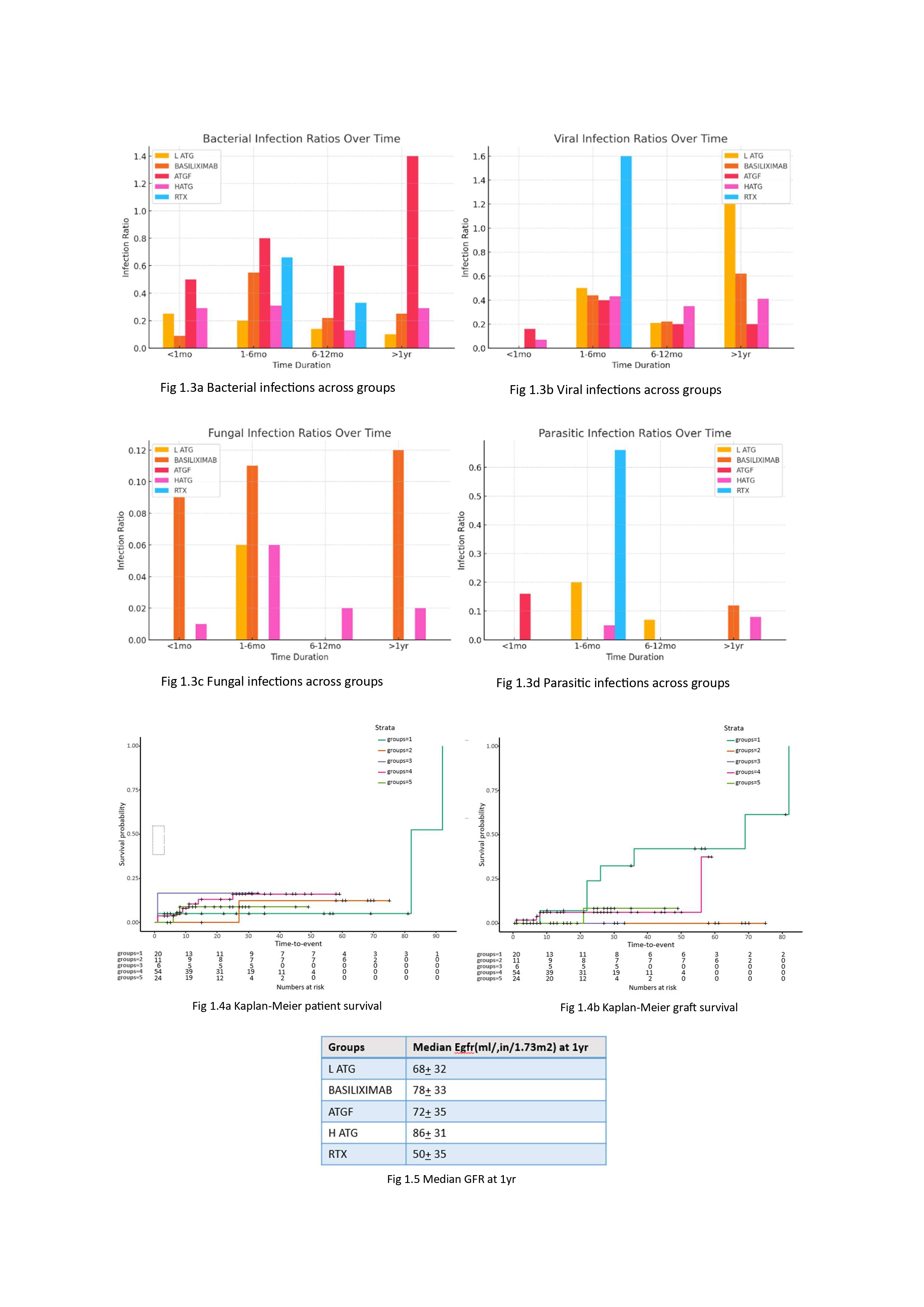
Results: Among the 94 recipients, induction groups included L-ATG (21.2%), basiliximab (11.7%), ATGF (6.3%), H-ATG (57.4%), and RTX (3.1%). Demographics were comparable across groups (Fig 1). The overall rejection rate was 28.6%. Basiliximab had the highest rejection rate within the first year (ABMR and TCMR) while RTX group had no rejections. L-ATG showed increased rejection rates beyond one year. There was no statistical difference in overall rejection rates (Fig. 1.1). The overall infection rate was 39.1%, lowest with basiliximab (30.9%), though differences across groups were not statistically significant (Fig. 1.2). UTIs (37.8%) were most common, followed by acute gastroenteritis (31.6%), with giardiasis being a notable parasitic infection. ATGF had significantly higher bacterial infection rates (p = 0.003), while viral infection rates were similar across groups (p = 0.87, Fig. 1.3a, 1.3b). CMV and BKV infections occurred in 18.4% and 12.4% of patients, respectively, with no significant group differences (p = 0.23). Incidence of TB was 1.7%. If infection control is the priority, Baisliximab is the best but has the highest rejection rate Kaplan-Meier analysis demonstrated comparable graft and patient survival across groups (Fig. 1.4a, 1.4b). Median eGFR at one year ranged from 50–86 mL/min/1.73m² (Fig. 1.5).
Conclusion: This study highlights the need to balance rejection and infection risks in pediatric kidney transplantation. Basiliximab had the highest early rejection rates, while L-ATG showed late-onset rejection. Despite these differences, graft survival and renal function remained comparable, emphasizing the importance of individualized immunosuppression strategies.
References:
[1] Induction immunosuppression
[2] Infections
[3] Rejections
[4] Basiliximab
[5] Graft survival
[6] Low dose ATG
