Medizinische Hochschule Hannover
Sex differences in endothelial cell senescence are independent of gonadal-hormones
Anna Quarder1, Khaoula Talbi 1, Anette Melk1.
1Interdisciplinary Transplantation, Medizinische Hochschule Hannover, Hannover, Germany
Introduction: Sex differences in cardiovascular diseases have been extensively described in the general population, with females considered hormonally protected. Recently, we showed that girls with chronic kidney disease after transplantation develop greater arterial stiffness than boys, challenging the assumed protective effect of the female sex. Endothelial dysfunction is amongst the earliest changes towards developing cardiovascular disease, with endothelial cell senescence playing a key role. Cellular senescence describes an irreversible cell cycle arrest and can be classified into replicative senescence (age-related) and stress-induced senescence (triggered by DNA damage). Previous studies have attributed sex-specific differences in senescence to the influence of sex hormones, while the role of sex chromosomes has been largely unexplored. This study analyzes whether sex-specific differences in endothelial cell senescence exist independent of hormonal influences and in the context of different stimuli (replication vs. DNA damage).
Methods: Cellular senescence was induced by replication or radiation in female and male HUVECs (n=7 each) cultured under hormone-free conditions. Senescence-associated-β-galactosidase (SA-β-Gal) staining, telomere length measurement, RT-qPCR of p14, p16, p21, and Crystal Violet proliferation assays were used to assess senescence. In replicative senescence, we sampled at multiple time points (passages 1-20) until senescence was reached, whereas in stress-induced senescence experiments, we collected samples on day 5 following irradiation.
Results: Female HUVECs showed a significantly longer replicative lifespan (119 ± 18 days) than male cells (88 ± 11 days) despite similar proliferation rates (Fig. 1a). Although early telomere lengths were comparable, male cells exhibited faster telomere shortening (Fig. 1b). On light microscopy, senescent cell morphology and SA-β-Gal staining (Fig. 1c) appeared at later passages in female cells, along with delayed expression of p16 (Fig. 1d), p21, and p14. Interestingly, the differences between female and male HUVECs following radiation exposure were opposite. Female HUVECs exhibited increased cellular senescence, as evidenced by increased SA-β-Gal staining and significantly higher expression of the cell cycle inhibitors p21, p16, and p14 compared to male HUVECs.
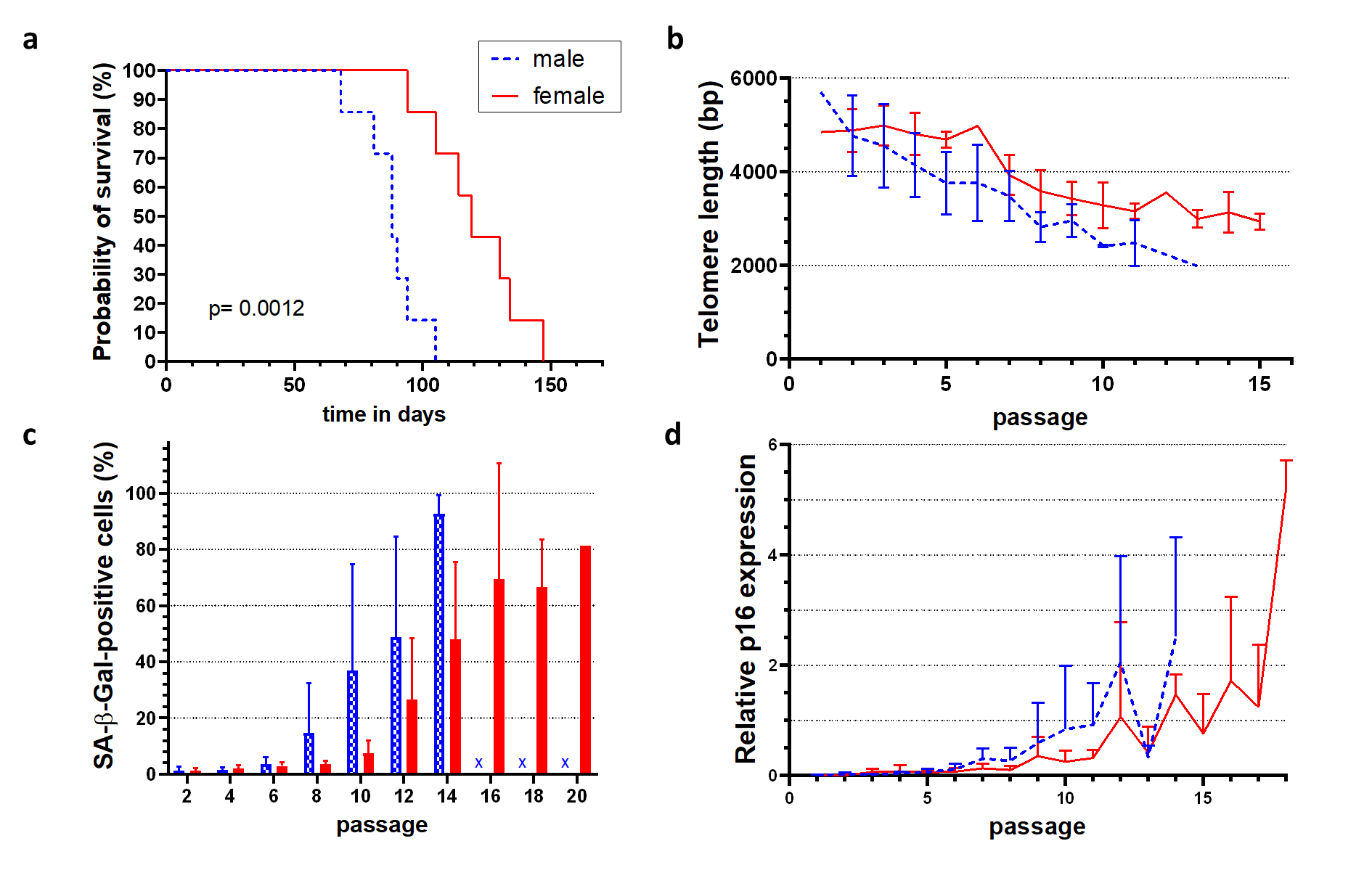
Conclusion: These findings reveal sex differences in endothelial senescence that occurred in vitro under hormone-free conditions, suggesting an influence of the chromosomal background. While male cells underwent faster replicative senescence, female cells were more susceptible to stress-induced senescence. Our data does not only highlight the potential influence of sex chromosomes in regulating vascular senescence but also differences in stimuli driving the development of senescence. In light of data indicating that the protective cardiovascular phenotype is lost in transplanted girls, our results are particularly significant.
References:
[1] Cellulare senescence
[2] Endothelial cells
[3] Sex differences
Lectures by Anna Quarder
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sun-21 09:15 - 10:45 |
Insights in immunology and immunosuppression | Sex differences in endothelial cell senescence are independent of gonadal-hormones | MOA 4 |
