Insights into the interplay between senescent cells and immune cells in the kidney microenvironment
Martin Jaros1, Hannah Bartmann1, Esther Beuke1, Anette Melk1.
1Interdisciplinary Transplantation, Hannover Medical School, Hannover , Germany
Introduction: Senescent cells occur with transplant-related stresses such as ischemia-reperfusion injury or rejection, but high blood pressure or hyperglycemia induce senescence as well. Senescent cells are in permanent cell cycle arrest, apoptosis resistant, and display pro-inflammatory and matrix-remodeling secretory profile. Cellular senescence affects the kidney’s ability to regenerate and contributes to graft dysfunction. The accumulation of senescent cells is not only a cellular stress response but also reflects dysfunctional immune surveillance, which should limit the build-up of senescence. This combination is particularly problematic in solid organ transplantation, which involves unavoidable stressors and also necessitates the use of immunosuppressant drugs.
This project aims to improve our understanding of immune surveillance by tracing the interaction between senescent cells and immune cells.
Methods: We utilized kidney sections from 30 aged mice (20-24 months) due to the proven presence of senescent cells. Kidneys from 15 young animals (3-5 months) served as controls. The immune landscape of these aged kidneys was characterized by immunohistochemical methods to describe the immune cell populations. Senescent cells were identified by cytochemical staining of senescence-associated beta-galactosidase (SA-β-Gal) and by in situ hybridization targeting p16INK4a, one of the main biomarkers of senescent cells. Additionally, gene expression profiling of kidney cortex samples was performed using Nanostring nCounter.
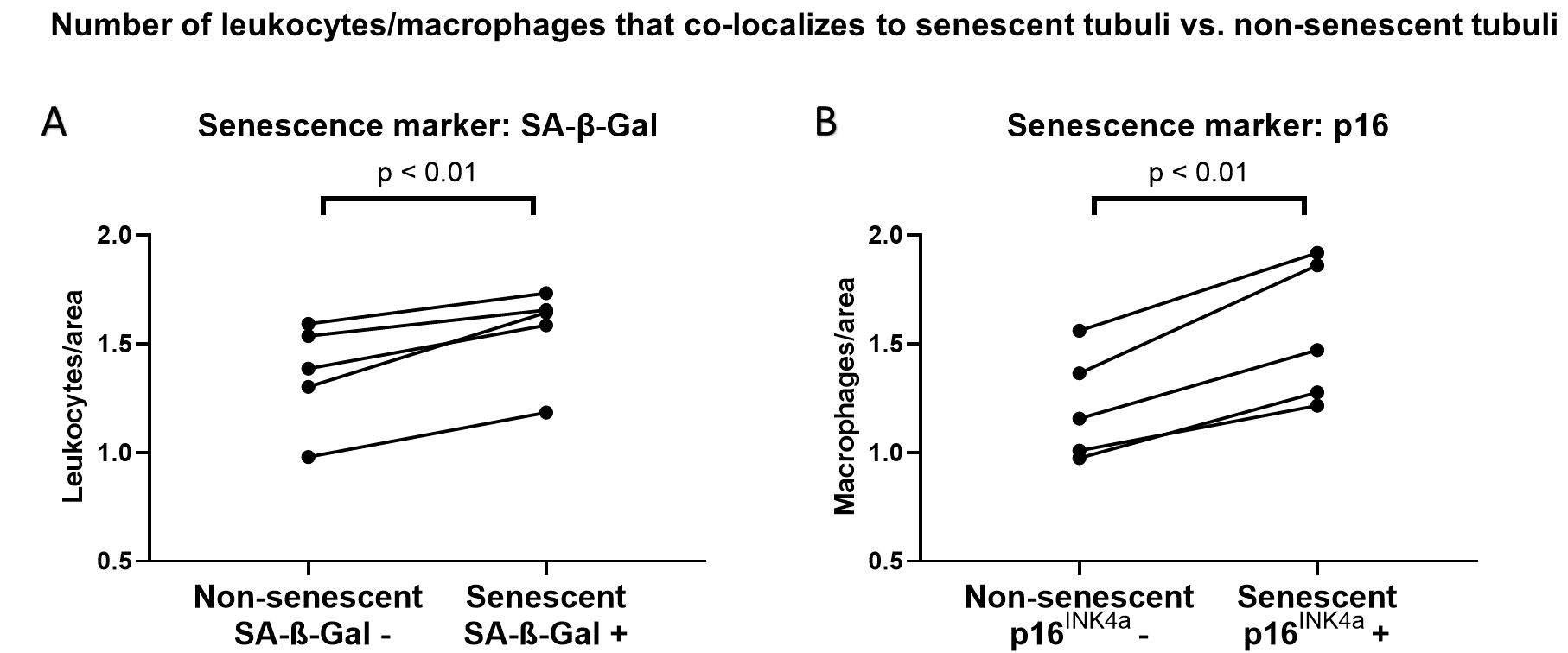
Results: As expected, aged kidneys exhibited a significantly larger number of senescent cells as demonstrated by increased p16INK4a staining (2.22 ± 1.23 vs. 0.22 ± 0.17 area per HPF in young mice) and increased SA-β-Gal staining (1.23 ± 0.74 vs. 0.02 ± 0.01 area per HPF in young mice). With older age, we found a significant increase in immune cell infiltration (9.99 ± 4.77 vs. 2.81 ± 0.97 cells per HPF in young mice), predominantly macrophages and T cells, concentrated in the kidney cortex. Accordingly, transcriptomic analysis revealed the upregulation of SASP genes with an increase in inflammatory and fibrosis-related pathways.
To better describe the alteration of a senescent kidney´s microenvironment, we performed colocalization analyses. We found that there was an increase in the number of immune cells in proximity to senescent tubular cells, detected by either SA-β-Gal (Fig. 1A) or by p16INK4a-ISH (Fig. 1B), compared to areas with no senescent cells.
Conclusion: These findings demonstrate a spatial connection between senescent and immune cells in light of a functioning immune response. In our current studies, we investigate whether senolysis, i.e., the removal of senescent cells, reduces immune cell infiltration. Understanding the interplay between senescence and immune response is crucial to improve transplant outcomes. Targeting senescent cells could offer a new strategy to enhance graft survival and long-term function.
References:
[1] Cellular senescence
[2] immune infiltration
[3] Chronic Inflammation
[4] Senolytic Treatment
[5] Kidney Allograft
Lectures by Martin Jaros
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sun-21 09:15 - 10:45 |
Insights in immunology and immunosuppression | Insights into the Interplay between Senescent Cells and Immune Cells in the Kidney Microenvironment | MOA 4 |
