University Hospital Essen
2D-shear wave elastography of pediatric liver transplants
Benas Prusinskas1, Metin Cetiner1, Ilja Finkelberg1, Kristina Kampmann1, Simone Kathemann1, Denisa Pilic1, Lars Pape1, Elke Lainka1.
1Department of Pediatrics II, University Children's Hospital, Essen, Germany
Background: In pediatric liver transplant recipients, accurate and non-invasive assessment of liver fibrosis is critical for long-term management and prognosis. Traditional liver biopsies, while still the gold standard, are invasive, carry risks, and are less feasible for regular monitoring. Two-dimensional shear wave elastography (2D-SWE) has emerged as a promising alternative, yet its diagnostic performance and influencing factors remain underexplored in pediatric transplant populations.
Objective: This study aimed to evaluate the diagnostic accuracy of 2D-SWE for liver fibrosis staging in pediatric liver transplant recipients and to identify biological and technical variables that may affect measurement reliability.
Methods: We conducted a retrospective analysis of 101 pediatric liver transplant recipients (age range: 1.3 – 17.9 years) who underwent both 2D-SWE and liver biopsy between November 2019 and July 2023 at University Children’s Hospital Essen. Histological staging (F0–F4) was performed using the Desmet classification. Liver stiffness values were compared to histological fibrosis stages, and receiver operating characteristic (ROC) curve analysis was used to determine diagnostic accuracy. We assessed the influence of biological (inflammation, cholestasis, steatosis) and technical (probe position, breath-holding, examiner experience) factors on measurement outcomes.
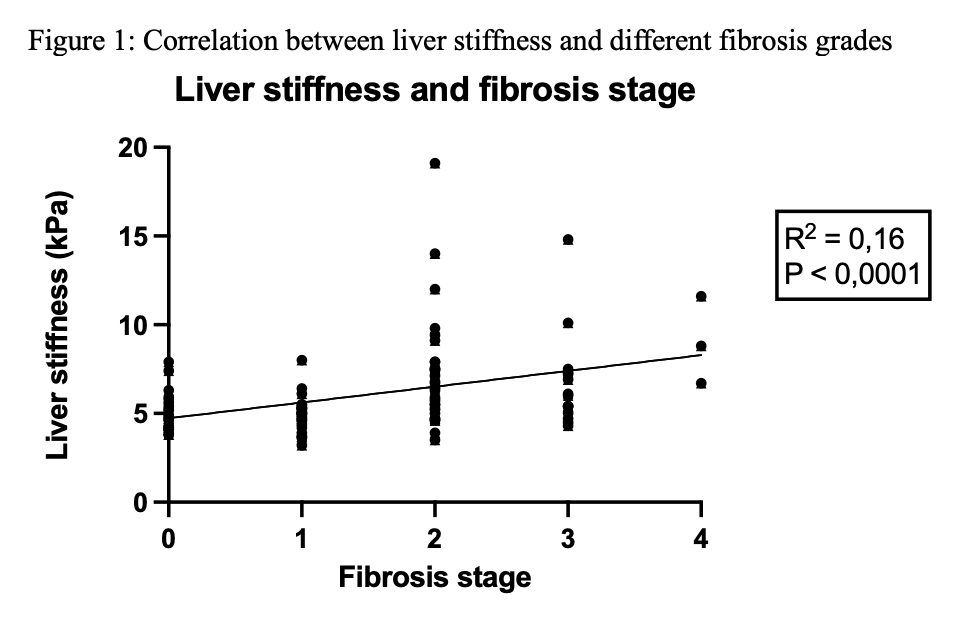
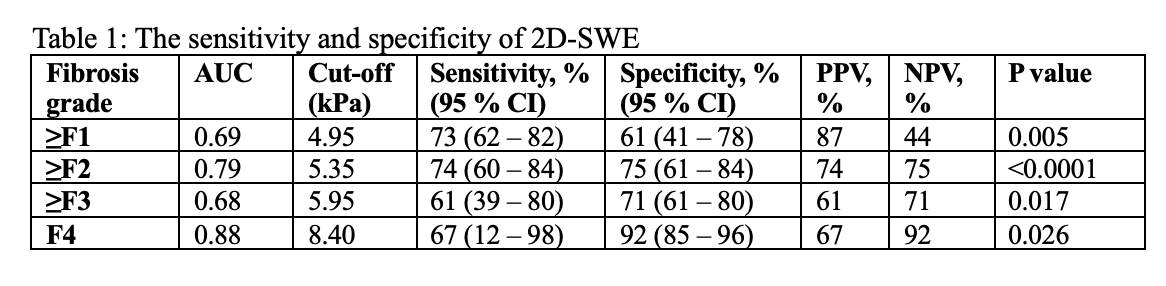
Results: 2D-SWE showed strong diagnostic performance with an AUC of 0.79 for ≥F2 and 0.88 for F4. The optimal cut-off for significant fibrosis (≥F2) was 5.35 kPa (sensitivity 74%, specificity 75%) and for cirrhosis (F4) was 8.40 kPa (sensitivity 67%, specificity 92%). Liver stiffness values were significantly elevated in patients with chronic cholestasis (7.1 vs. 5.3 kPa, p=0.01) and moderate inflammation (G2) (7.1 vs. 5.3 kPa, p=0.05). Steatosis had no significant effect (p=0.85). Technical factors, including breath-hold ability, probe position, and examiner experience, did not significantly influence results.
Conclusion: 2D-SWE is a reliable, non-invasive tool for evaluating liver fibrosis in pediatric liver transplant recipients and offers a valuable alternative to liver biopsies. Its diagnostic performance is high, especially for detecting advanced fibrosis. While technical variables showed minimal influence on measurements, biological factors such as inflammation and cholestasis should be considered during interpretation. Standardized examination protocols further enhance reproducibility, making 2D-SWE suitable for routine clinical use in post-transplant surveillance.
Lectures by Benas Prusinskas
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Fri-19 11:05 - 12:05 |
Breaking down barriers to transplant in low resource settings | Panel discussion on the challenges of access to transplant and innovative solutions: Baltic perspective | MOA 3 |
|
Sun-21 09:15 - 10:45 |
Complications of pediatric liver transplantation | 2D-Shear Wave Elastography of Pediatric Liver Transplants | MOA 5 |
