Maria Julia Minetto, Argentina
Garrahan Hospital
HLA molecular mismatch as a predictor of rejection and donor-specific antibodies in pediatric liver transplantation
Guido Trezeguet Renatti1,2, Agostina Arrigone1, Belen Cabral1, Julia Minetto1, Florencia Degrave1, Santiago Cervio1, Gabriela Aboud1, Hayellen Reijenstein1, Leandro Lauferman1, Agustina Jacobo Dillon1, Diego Aredes1, Ivone Malla1, Stefania Conde1, Catalina Costas1, Andrea Bosaleh1, Daniela Fernandez Souto1, María Fernanda Yaunguzian3, Pablo Galarza3, Marcelo Dip1, Oscar Imventarza1, Cecilia Gamba1, Esteban Halac1, Paula Schaiquevich1,2.
1Hospital de Pediatría JP Garrahan, Buenos Aires, Argentina; 2CONICET, Buenos Aires, Argentina; 3INCUCAI, Buenos Aires, Argentina
Introduction: Alloactivation-induced immunological injury to the liver allograft remains a major threat to long-term allograft survival. Identifying risk factors for allograft injury is essential to preserve its function. Previous studies at our center demonstrated the impact of tacrolimus variability on the risk of developing biopsy-proven acute rejection (BPAR). We also identified HLA-DQ eplet mismatch (eMM) as a risk factor for BPAR in preliminary studies, though its role in donor-specific antibodies (DSA) development remains unclear in liver transplantation. This study aimed to identify risk factors for BPAR and de-novo DSA (dnDSA) in pediatric liver transplant patients.
Methods: Patients transplanted between 2018 and 2023 were included and prospectively followed. Donor/recipient pairs were HLA-typed by NGS; HLA eMM were quantified using the HLA-Matchmaker algorithm, and the PIRCHE-II scores were also calculated. Histopathological outcomes were obtained from surveillance and for-cause biopsies. Tacrolimus variability was quantified as tortuosity. Serum samples collected at scheduled timepoints were analyzed for dnDSA using LIFECODES Single Antigen Assays. Demographic, peri-transplant, clinical, and pharmacological variables were analyzed in Cox regression models to identify predictors of BPAR and dnDSA.
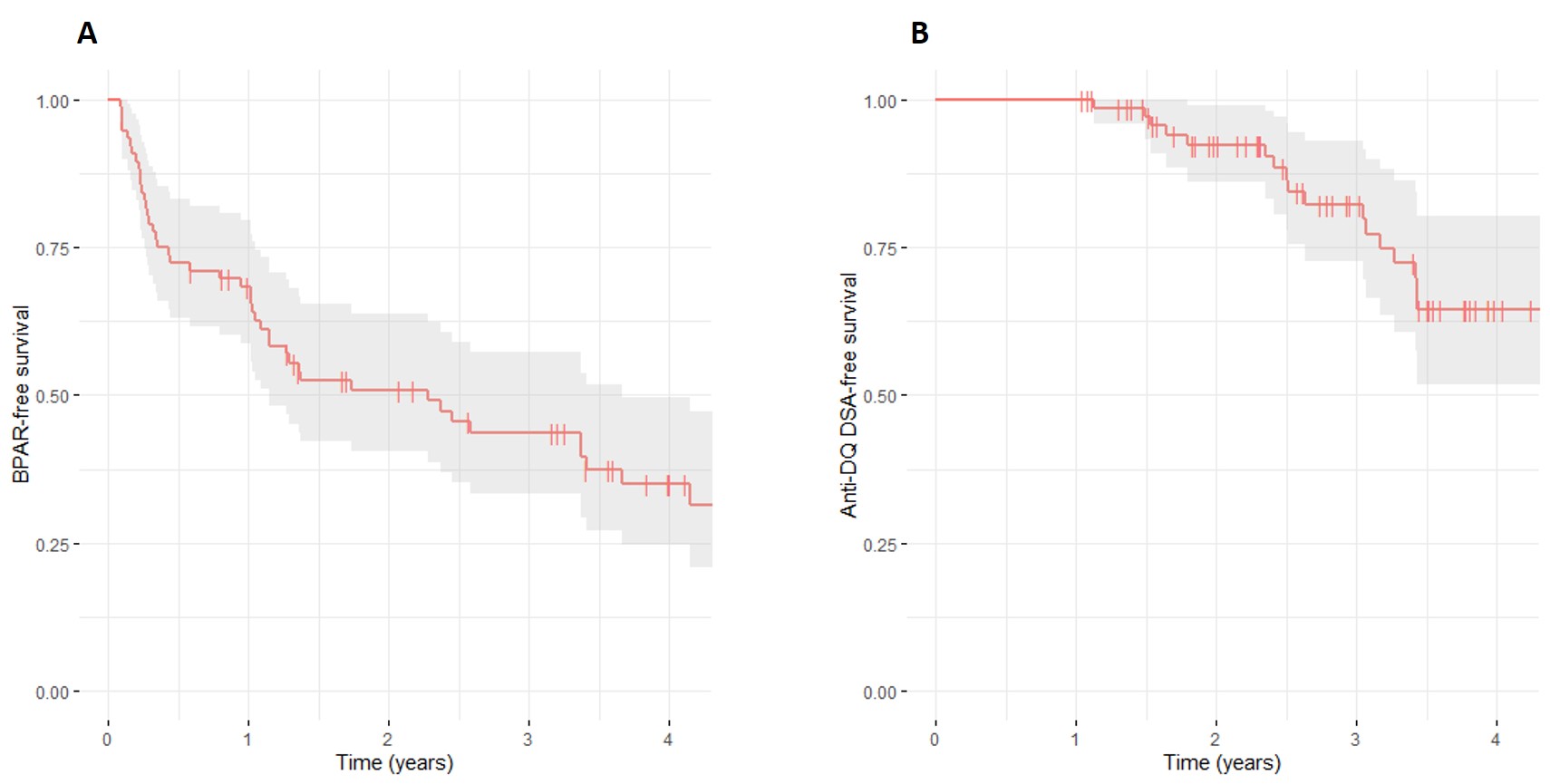
Results: Of 163 patients recruited, 76 had available high-resolution HLA typing data. BPAR-free survival at 1 year post-transplant was 68.3% (95%CI: 58.6-79.6), but only 35.0% (95%CI: 24.6-49.6) of the patients were free of rejection at 4 years post-transplant (Figure 1). Independent risk factors for BPAR included tacrolimus tortuosity (HR 8.80, 95%CI: 3.67-21.10, p<0.001), HLA-DQ antibody-verified (ab) eMM (HR 1.21, 95%CI: 1.06-1.38; p=0.006), and receiving an allograft from a deceased donor (HR 2.42, 95%CI: 1.27-4.59; p=0.007).
Among 74 patients with available single-antigen assay results, 26 developed dnDSA: 2 patients had anti-HLA class I, 22 had anti-HLA class II, and 2 had anti-HLA class I and II antibodies. The most frequent dnDSA was anti-DQ (n=18). Anti-DQ DSA-free survival at 1 year post-transplant was 100% (95%CI: 100.0-100.0) but only 58.7% (95%CI: 46.1-74.8) were free of anti-DQ DSA at 4 years (Figure 1). Independent risk factors for developing anti-DQ DSA included HLA-DQ ab eMM (HR 1.46, 95%CI: 1.18-1.80, p<0.001), and prior BPAR event (HR 5.04, 95%CI: 1.10-23.11, p=0.037).
Conclusion: In this large cohort of pediatric liver transplant patients, we emphasize the role of HLA molecular mismatch in assessing the immunological risk. Variability in tacrolimus trough levels, HLA-DQ ab eMM, and donor type were independent predictors of BPAR, while HLA-DQ ab eMM and prior BPAR events independently predicted the development of anti-DQ DSA. These findings underscore the value of integrating pharmacological and immunogenetic markers to enable individualized surveillance and optimize alloimmune control in children.
Lectures by Maria Julia Minetto
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Fri-19 13:35 - 15:05 |
Indications for pediatric liver transplantation | Facing the issue of acute-on-chronic liver failure in pediatric biliary atresia: a prognostic and therapeutic challenge | MOA 5 |
|
Sun-21 09:15 - 10:45 |
Complications of pediatric liver transplantation | HLA molecular mismatch as a predictor of rejection and donor-specific antibodies in pediatric liver transplantation | MOA 5 |
|
Thu-18 17:00 - 18:00 |
Liver/Intestine Posters - from P1.35 to P1.53 | Expanding the donor pool for pediatric liver transplantation with living donor left side technical variant grafts | MOA 10 (Exhibit Area) |
