Medizinische Hochschule Hannover
Identifying high-risk hearts after pediatric kidney transplantation: Linking diastolic dysfunction to myocardial fibrosis
Tim Ubenauf1, Jeannine von der Born1, Rizky Sugianto1, Carl Grabitz1, Elena Lehmann1, Nele Kanzelmeyer1, Nigar Babazade2, Samir Sarikouch3, Diane Renz2, Bernhard Schmidt4, Anette Melk1.
1Department of Pediatric Kidney, Liver, Metabolic and Neurological Diseases, Hannover Medical School, Hannover, Germany; 2Institute of Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany; 3Department of Cardiothoracic, Transplantation and Vascular Surgery, Hannover Medical School, Hannover, Germany; 4Department of Nephrology and Hypertension, Hannover Medical School, Hannover, Germany
Sudden cardiac death is one of the leading causes of death in patients with chronic kidney disease (CKD) following pediatric kidney transplantation (KTx). We have recently shown cardiac remodeling with myocardial fibrosis in a significant proportion of young KTx recipients detected by cardiovascular magnetic resonance (CMR) imaging. While CMR cannot be performed in all patients, there is the need to define standardized diagnostic procedures for identifying children at risk, which have not been established. This study aimed to characterize functional alterations in young KTx recipients using echocardiography and to investigate their associations with structural cardiac damage.
46 KTx recipients (mean age 16.0±3.5 years) underwent comprehensive echocardiography: ejection fraction (EF), mitral valve inflow (E-, A-wave), TDI: (e'), E/e'-ratio, isovolumetric relaxation time (IVRT), pulmonary venous flow (PVFsys, PVFdia), atrial reversal (PVF-AR), left atrial volume index (LAVI) and left ventricular mass z-score (LVMz). The KTx recipients and 46 age- and sex-matched healthy controls underwent non-contrast CMR to asssess native T1 time (nT1), a marker for myocardial fibrosis, left ventricular mass index z-score (LVMIz), EF and global longitudinal strain (GLS). Multivariable linear regression analyses (co-variates: age, sex, height) were used to show associations between echocardiographic and CMR parameters.
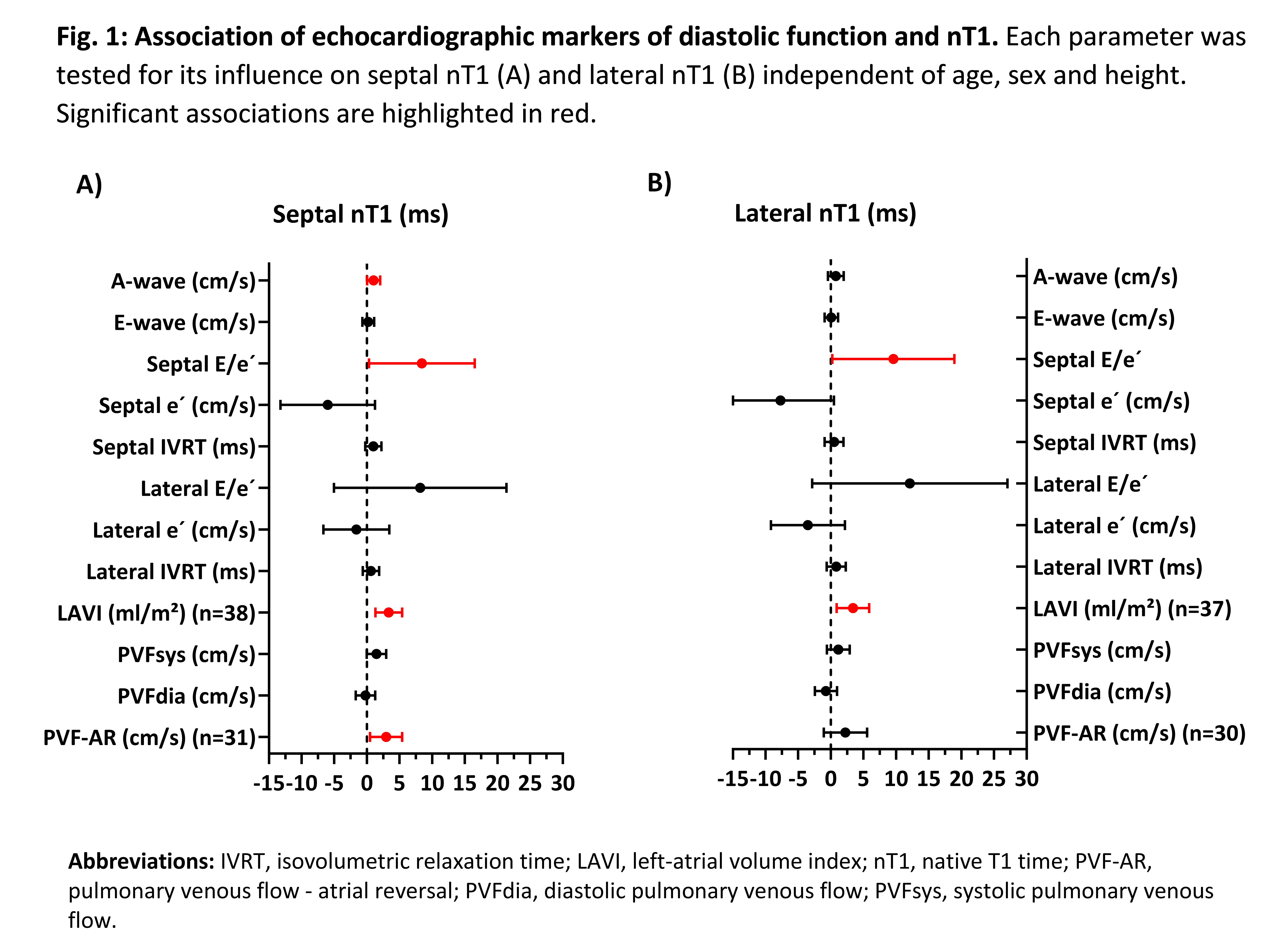
On echo, 90% (n=40) of the KTx recipients showed in two or more diastolic parameters values out of the age-specific normal range. EF was preserved in all patients; 11% (n=5) displayed left ventricluar hypertrophy (LVH). Septal and lateral nT1 (assessed by CMR) was higher in KTx recipients compared to healthy controls (septal: 1198±49ms vs. 1155±23ms, p<0.0001; lateral: 1141±57ms vs. 1109±37ms, p=0.003). Significant associations of four echo-parameters with septal nT1 were found (Fig. 1A) and included: septal E/e' (ß=8.415, p=0.042), A-wave (ß=1.037, p=0.045), LAVI (ß=3.347, p=0.002) and PVF-AR (ß=2.945, p=0.021). Two echo-parameters were associated with lateral nT1 (Fig. 1B; septal E/e': ß=9.590, p=0.045 and LAVI: ß=3.393, p=0.009). 7 of 8 KTx recipients with elevated lateral nT1 also had an elevated septal nT1, on echo septal E/e' values were above the age-specific range in all 8 KTx recipients.
Young KTx recipients display a high prevalence of diastolic dysfunction with preserved systolic function on echo and these changes are associated with structural changes reflecting myocardial fibrosis. Our findings suggest that a large number of children after KTx suffer from heart failure with preserved EF (HFpEF)– an entity not yet defined in children. In case of diastolic dysfunction, a CMR seems justified and allows an improved risk stratification in this high-risk patient group. Longitudinal studies are needed to understand how these changes evolve over time to allow not only better prediction, but the implementation of an early heart failure therapy.
Supported by KlinStrucMed Program and Hannover Medical School, funded by ‘Dr. August und Erika Appenrodt Stiftung’.
References:
[1] Myocardial Fibrosis
[2] Diastolic Dysfunction
[3] Cardiovascular Magnetic Resonance
[4] Echocardiography
[5] Sudden Cardiac Death
[6] Risk Stratification
[7] Long Term Outcome
Lectures by Tim A. Ubenauf
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sun-21 09:15 - 10:45 |
Monitoring after pediatric kidney transplant | Identifying high-risk hearts after pediatric kidney transplantation: linking diastolic dysfunction to myocardial fibrosis | MOA 6 |
