ABO incompatible deceased donor liver transplants in infants (age <1 Year): A single-center experience
Heli Bhatt1, Andrew Adams1, Sarah Jane Schwarzenberg1, Srinath Chinnakotla1.
1Surgery, University of Minnesota, Minneapolis, MN, United States
Background: The waitlist mortality for children < 1year is currently the highest, listed at 20 per 100 years of waiting time in USA. ABO incompatible (ABOi) liver transplantation is one of the strategies to increase transplantation rate in infants, however it is utilized only in 5.6% of infants. The goals of this study were twofold. First, we analyzed the waiting time, The waitlist Mortality, transplantation rate in children that were listed for ABOi and second: evaluated the transplant outcomes of patients that received ABOi livers compared to ABOc livers.
Methods: At our center, all children < 1 year are listed for ABOi liver transplants in the absence of a potential living donor and if the anti abo titers are less than 1:8, no plasmapheresis was used. Thymoglobulin is used for induction.
Results: Of the 19 pediatric patients that received ABOi liver transplants at our center between 2010- 2024; 16 of them were <1 year of age and formed the study group. They were compared to 56 ABOc liver transplants in children <1 year performed at our center during the same time frame. The median age of ABOi group was 6.7± 2.2 months compared to 8.0 ± 2.7 months for ABOc group. The commonest indication for transplantation was biliary atresia. The median waiting time in the ABOi group was 53 days compared to 68 days in the ABOc group. ABOi group utilized AB or B blood type livers 62% (10 livers) more often compared to none in the ABOc group. The commonest graft type was a whole liver in both groups. 33% in the ABOc group received partial grafts compared to 25% in the ABOi group. No patient required plasmapheresis, and the post-transplant complications are listed in Table 1.
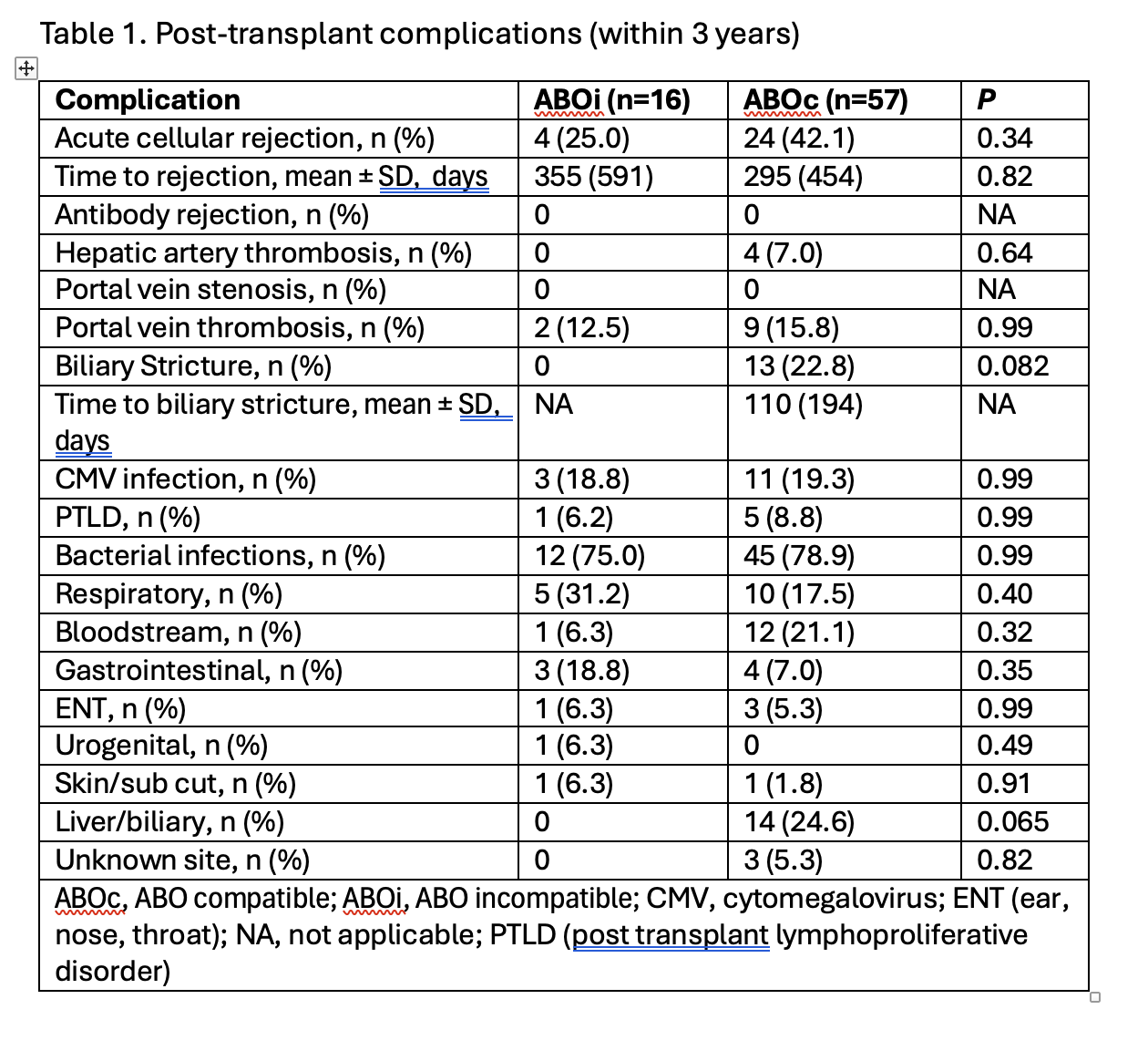 .
.
The patient and graft survival are shown in Figure 1, similar between groups. Review of UNOS data during the same time frame showed The median wait times for patients <1 year for blood types A, B, AB, and) were 96 days, 109 days, 82 days and 126 respectively. The patients listed to accept ABOi livers had a shorter waiting time. The no utilization of blood type AB livers and B livers among donors less <18 years in USA during the same time was 5.93% and 5.9% respectively.
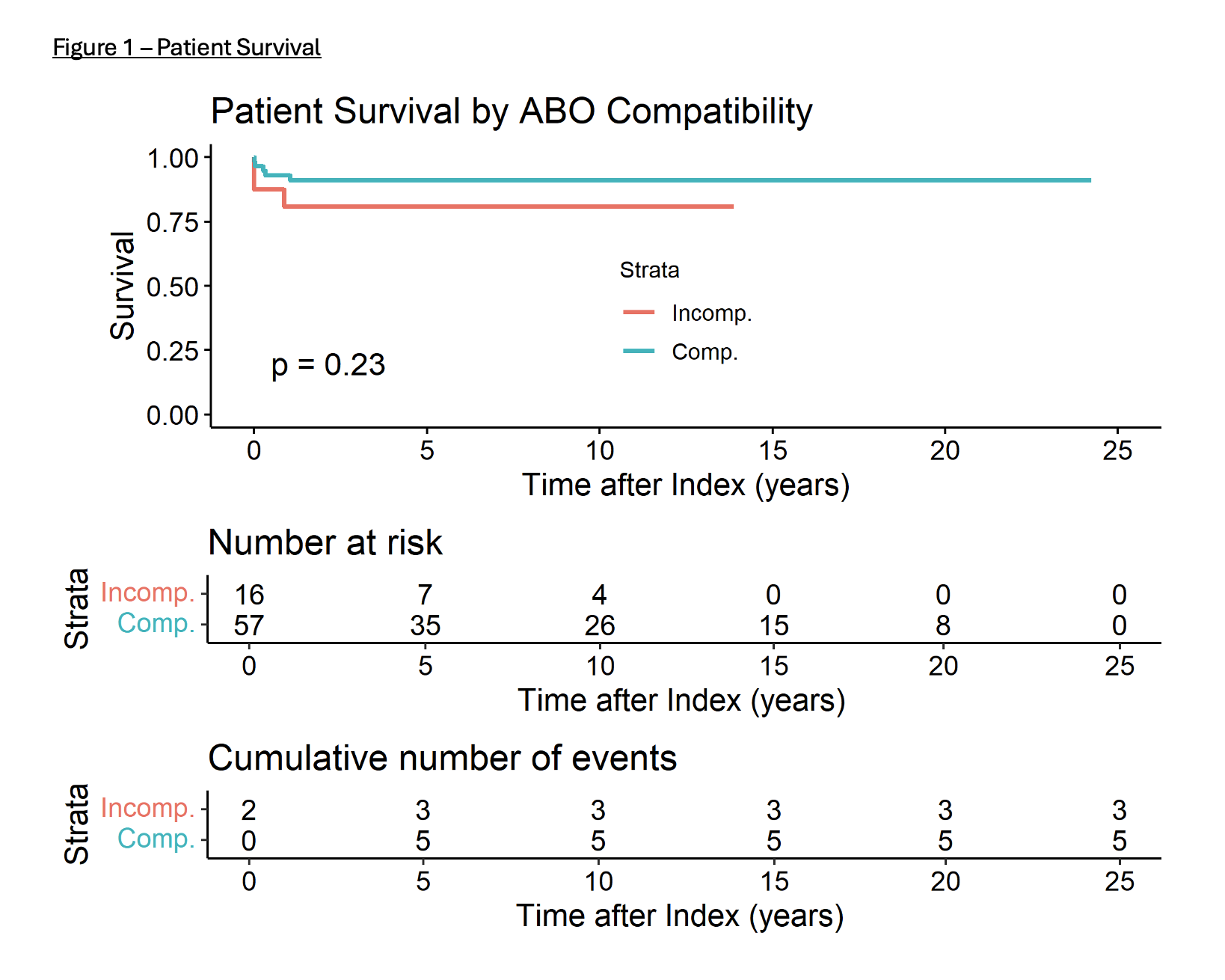
Conclusions: Our study shows that children <1 year that receive ABOi liver transplants have similar outcomes to those that receive ABOc transplants. A child listed to receive ABOi has a shorter waiting time and almost no waitlist mortality. This practice also results in increased utilization of AB-type livers, reducing the discard rate. These data support changing the current UNOS policy to allow listing all infants to accept all blood types.
References:
[1] liver transplant
