Douglas G. Farmer, United States
Chief
Division of Liver and Pancreas Transplantation
UCLA Health Department of Surgery
Excellent outcomes with high-risk pediatric hepatoblastoma: A detailed analysis of a large, single center experience
Valeria Ripa1, Robert S Venick2, Samer Ebaid1, Noah Federman1, Nicole Charland1, Caitlin J Thornley1, Jonathan Jou1, Brandon Pearson1, Vatche G Agopian1, Fady M Kaldas1, Ronald W Busuttil1, Suzanne V McDiarmid2, Douglas G Farmer1.
1Surgery, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States; 2Pediatric Gastroenterology, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States
Pediatric .
Introduction: Hepatoblastoma (HBA) is an uncommon pediatric malignancy that can be treated with liver transplantation (LTx). There are limited reports on the outcomes after LT for HBA. Herein, we review our large, single center experience with HBA and LT.
Methods: A retrospective review of a prospectively maintained database was conducted. All children who underwent LT with the diagnosis of HBA were included. This study represented a 40 year experience (1984-2024). Detailed data was extracted regarding the presenting size and extent of HBA as well as surgical and non-surgical treatments. For LT, details regarding the procedure and outcomes were also included. Study end points included survival and disease recurrence. Statistical differences were determined by standard statistical testing with significance at P <0.05.
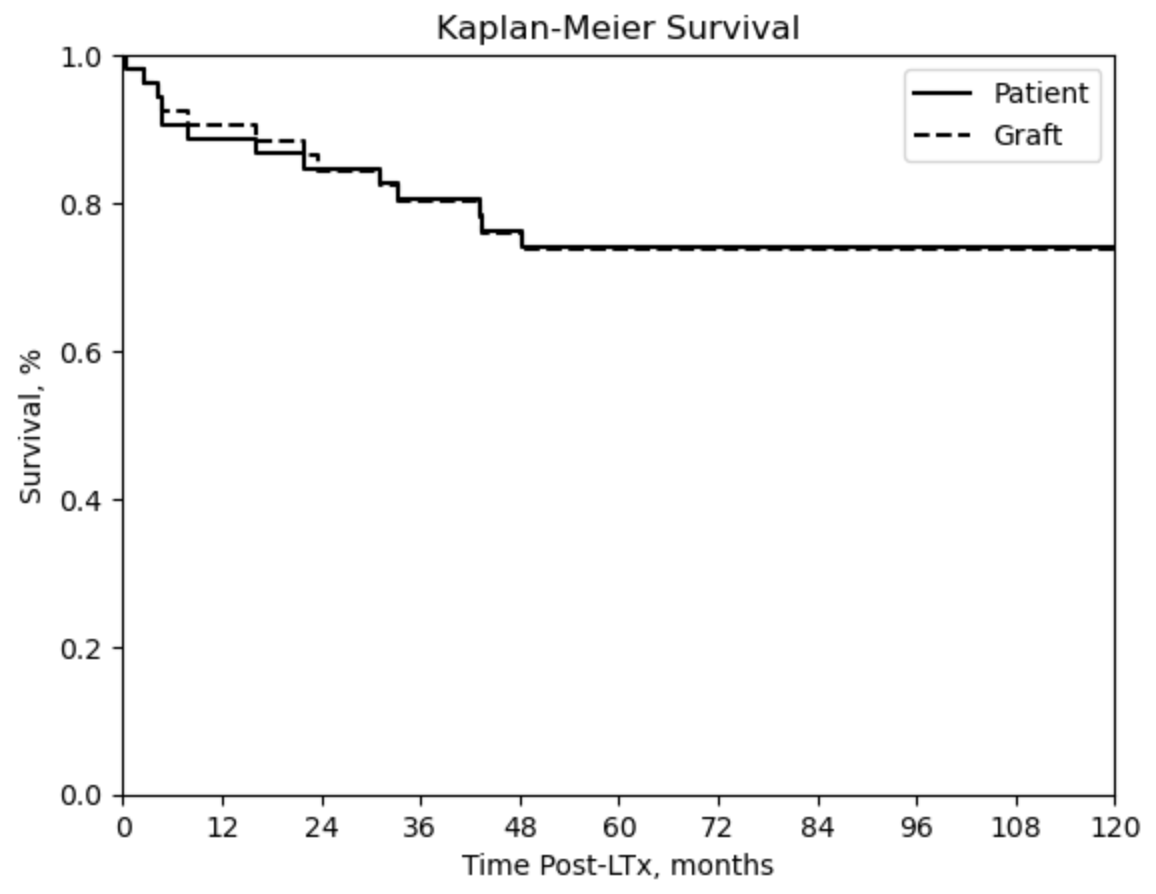
Results: A total of 55 patients were included, with the majority being male (58%) and Hispanic (40%). The mean age at diagnosis was 36 months, and the mean AFP level at diagnosis was 569,355 ng/mL. PRETEXT staging was: stage II (9%), stage III (44%), and stage IV (47%). All patients except one received chemotherapy, and 15 underwent surgical resection prior to LT. The mean wait time from diagnosis to transplantation was 10 months. Metastatic disease was identified preLT in 5 patients, all exhibiting pulmonary involvement. Explant pathology revealed positive lymph nodes (n=3), vascular invasion (n=16), and tumor invasion or rupture (n=2). Post-transplant complications included HBL recurrence (20%), hepatic artery thrombosis (HAT) (11%), and biliary stricture (20%). Patient survival rates at 1-, 5-, and 10-year post-LT were 89%, 74%, and 74% respectively. Graft survival rates at 1-, 5-, and 10-year post-LT were 91%, 74%, and 74%, respectively, as depicted in Figure 1. Covariates identified as statistically significant predictors of tumor recurrence included longer wait time from diagnosis to transplantation (p=0.02), prior resection (p=0.01), large tumor size (p=0.02), and vascular involvement (p=0.02). Mortality was predominantly attributed to tumor recurrence.
Conclusion: This study constitutes one of the largest single-center experiences with LTx in pediatric patients with HBA. Our findings demonstrate that excellent survival outcomes are achievable in this high-risk population. Several key variables associated with an elevated risk of tumor recurrence and subsequent mortality were identified. The most significant predictors suggest that resection should be avoided in cases with a high likelihood of recurrence. Notably, metastatic disease at diagnosis was not associated with increased tumor recurrence, indicating that aggressive therapeutic strategies should be pursued. The HAT rate in this cohort was threefold higher than in our broader pediatric LTx population, possibly linked to a thrombophilic state induced by malignancy and chemotherapy.
None.
References:
[1] Liver transplant
[2] Hepatoblastoma
[3] Pediatric transplant
[4] Allograft survival
[5] Cancer recurrence
[6] Pediatric malignancy
[7] Transplant for hepatoblastoma
[8] Biliary stricture
[9] Graft rejection
[10] Pediatric survival
Lectures by Douglas G. Farmer
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Fri-19 13:35 - 15:05 |
Indications for pediatric liver transplantation | Excellent outcomes with high-risk pediatric hepatoblastoma: a detailed analysis of a large, single center experience | MOA 5 |
