Head Section: Pediatric Transplant Nephrologist
Pediatric kidney transplant section
King Fahad Specialist Hospital-Dammam
Utility of de novo donor-specific antibody-driven protocol kidney biopsy in pediatric kidney transplantaion
Alanoud Alshami1, Ahmed AA Azzam1, Hebattallah HB Bahbah1, Ammar AH Hamed1, Nareen NM Mohammed1.
1Pediatric Nephrology and Kidney Transplant, King Fahad Specialist Hospital-Dammam, Dammam, Saudi Arabia
Introduction: The development of de novo donor-specific antibodies (dnDSA) following kidney transplantation is closely associated with the onset of antibody-mediated rejection (AMR), which significantly affects graft survival.
Methods: In 2015, a standardized protocol for monitoring dnDSA was established, involving assessments at 6 months and 1 year post-transplant, followed by annual evaluations and additional assessments as indicated. Kidney biopsies were performed, and dnDSA was treated when the mean fluorescence intensity (MFI) of dnDSA reached or exceeded 3000,
The study included 137 pediatric patients under 18 years of age who underwent transplantation between January 2015 and December 2023. A control group comprised 67 patients who were transplanted between January 2008 and December 2014.
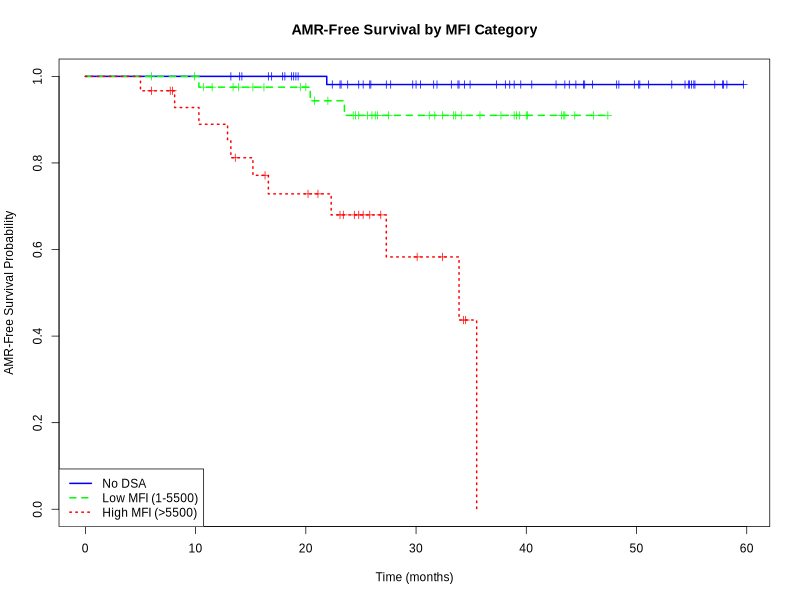
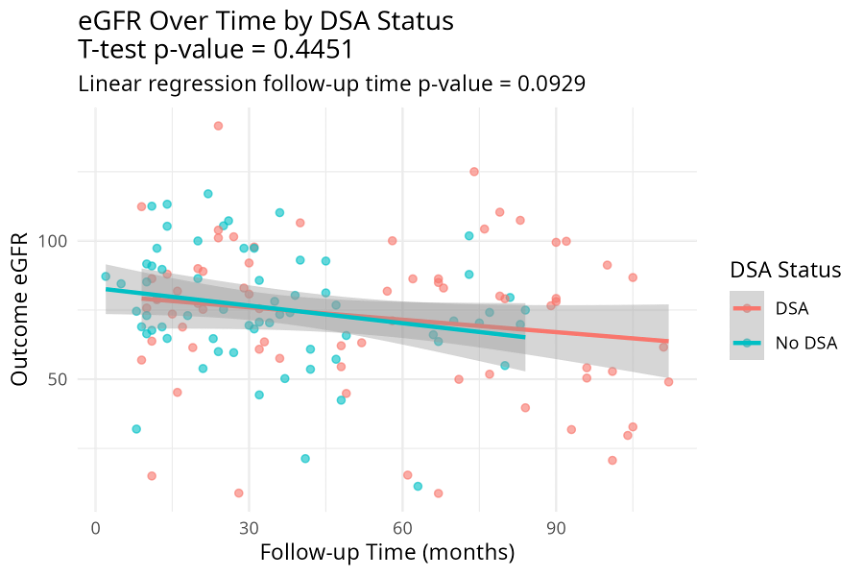
Results: dnDSA was identified in 72 patients, with with an incidence rate of 52.6%, and a median occurrence of 16.5 months (IQR, 8-30.25 months). Of the 72 dnDSA-positive patients, 60 exhibited normal baseline creatinine levels at the time of dnDSA detection. Among these 60 patients, 42 underwent kidney biopsy, with a median peak MFI at the time of biopsy of 8950 (IQR, 3900-17950). DQ was the immune dominant dnDSA, accounting for 37.5% of the cases, followed by DR in 6.7%. Forty percent of the biopsies showed no significant changes. Subclinical antibody-mediated rejection (sABMR) was identified in 20 patients (47.6%), including 11 with pure active ABMR (26.2%), 3 with mixed rejection (7%), and 1 with chronic active ABMR (2.4%). Five additional patients were classified as having probable ABMR (11.9%) based on the 2022 Banff classification, and five (12%) exhibited subclinical acute cellular-mediated rejection (sACR) . All patents with sABMR were treated. Multivariate analysis identified significant independent predictors of AMR, including high MFI (>5500) (OR 27.08, 95% CI 1.63 - 857.86, p=0.0280), HLA mismatches ≥ 4 (OR 0.12, 95% CI 0.02 - 0.62, p=0.0193), and the presence of DQ antibodies (OR 9.38, 95% CI 1.59 - 88.72, p=0.0247). ROC curve analysis demonstrated that an MFI of 5030 was highly predictive of AMR, with an area under the curve (AUC) of 0.78, high sensitivity (94.4%), and moderate specificity (58.3%). The rate of decline in estimated glomerular filtration rate (eGFR) for the DSA-positive group was -2.13 ml/min/1.73 m2 per year, which was comparable to the DSA-negative group ( p-value = 0.44510). However, this decline was significantly lower than that observed in the control group, which was -5.34 ml/min/1.73 m2 per year (p-value = 0.0013).
Conclusion: dnDSA-drivn biopsies detected 47.6% cases of sABMR and 12% cases of sACR. Regular dnDSA monitoring paired with kidney biopsy performance may provide a more comprehensive assessment of graft status in children. This integrated approach supports early intervention and can potentially optimize long-term graft survival
References:
[1] Graft rejection
[2] kidney biopsy
[3] dnDSA
Lectures by Alanoud Alshami
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Fri-19 07:00 - 07:50 |
Understanding rejection in pediatric kidney transplant | Utility of De Novo Donor-Specific Antibody-Driven Protocol Kidney Biopsy in Pediatric Kidney Transplantaion | MOA 5 |
|
Sat-20 15:30 - 16:30 |
Important recipient considerations in pediatric kidney | Breaking the Weight Boundaries: : Exceptional Kidney Transplant Outcomes in Children <12 kg and <10 kg. Center Experience | MOA 5 |
|
Thu-18 17:00 - 18:00 |
Kidney Posters - from P1.1 to P1.32 | Bridging the gap: Navigating donor-recipient size mismatch in pediatric renal transplants | MOA 10 (Exhibit Area) |
