Carolina Magalhães Magalhães Costa, Brazil
Hospital Sírio-Libanês
Basiliximab induction and steroid-free tacrolimus vs. tacrolimus-corticosteroid therapy in pediatric liver transplantation: A systematic review and meta-analysis
Carolina Costa1, Elizabet Taylor Pimenta Weba2, Alexandros Páris de Mesquita Ipácio2, Karlos Daniell Araújo dos Santos3, Eduardo Antunes da Fonseca1, João Seda Neto1.
1Hepatology and Liver Transplantation, Hospital Sírio-Libanês, São Paulo, Brazil; 2Medical Student, State University of Maranhão Tocantine Region, Tocantis, Brazil; 3Medical Student, Federal University of Roraima, Raraima, Brazil
Introduction: Liver transplantation is the gold standard treatment for end-stage liver disease in children, offering excellent survival rates. However, graft rejection and immunosuppression (IS) side effects remain significant causes of morbidity. Traditional IS regimens include corticosteroids for both induction and maintenance. Yet, the potential benefits and risks of a steroid-free postoperative approach using basiliximab remain unclear, particularly in pediatric patients, where minimizing steroid-related side effects is crucial.
Methods: We conducted a systematic review and meta-analysis comparing tacrolimus with basiliximab against tacrolimus with steroids in pediatric liver transplant patients. Statistical analysis was performed using R (version 4.4.1). Risk ratios (RRs) were calculated for dichotomous outcomes, and mean differences (MDs) for continuous outcomes. A random-effects model was used to estimate 95% confidence intervals (CIs).
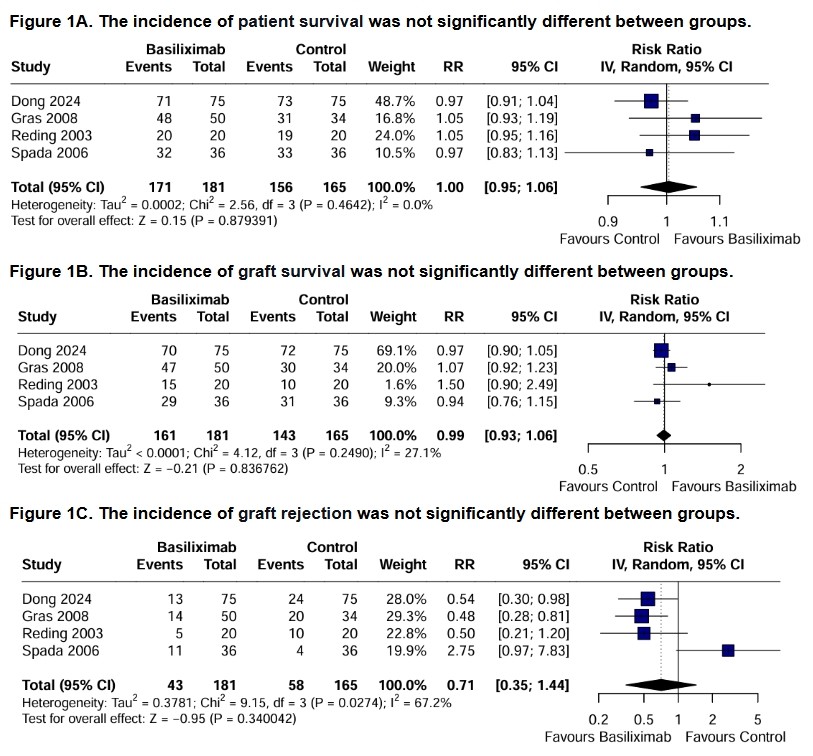
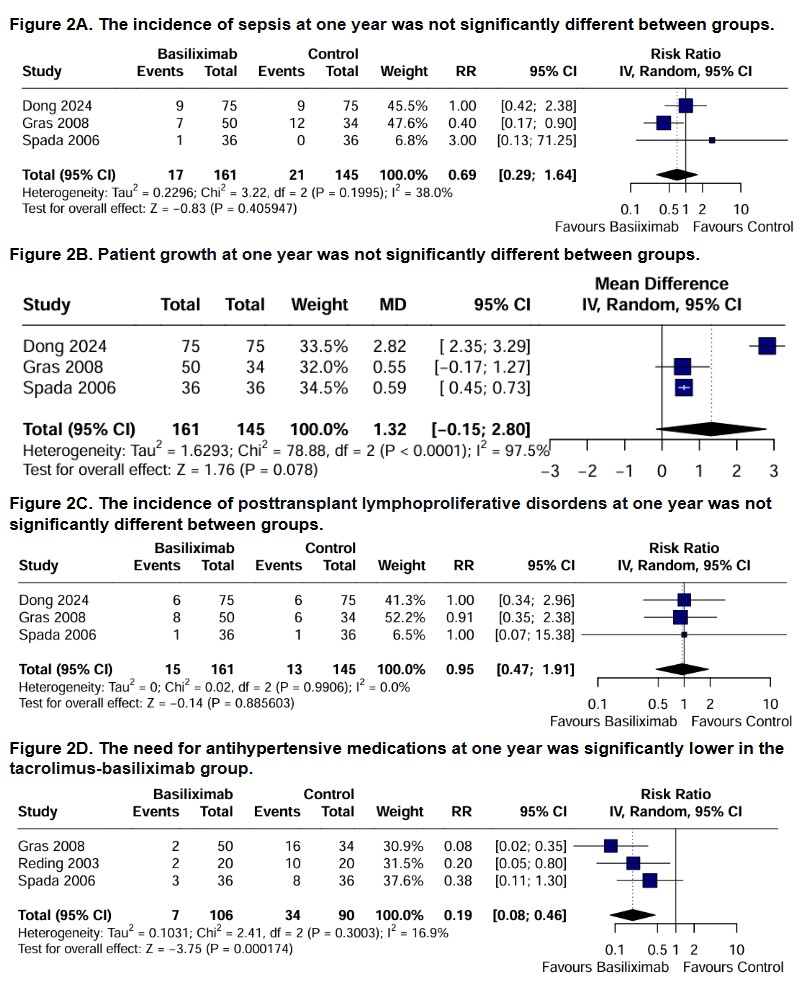
Results: Four studies and 346 patients were included, of whom 181 (52.3%) received a steroid-free regimen. The population was predominantly female (51.4%) and had a mean (SD) age of 4.26 (2.42) years. Follow-up ranged from 12 to 36 months. Pooled data showed no significant differences between groups in patient survival (RR 1.00; 95% CI 0.95 to 1.06; p = 0.879; figure 1A), graft survival (RR 0.99; 95% CI 0.93 to 1.06; p = 0.837; figure 1B) and graft rejection (RR 0.71; 95% CI 0.35 to 1.44; p = 0.340; figure 1C). Similarly, no significant differences were observed at one year for sepsis (RR 0.69; 95% CI 0.29 to 1.64; p = 0.406; figure 2A), patient growth (MD 1.32; 95% CI -0.15 to 2.80; p = 0.078; figure 2B) and posttransplant lymphoproliferative disorders (RR 0.95; 95% CI 0.47 to 1.91; p = 0.886; figure 2C). However, the tacrolimus-basiliximab group was associated with a significant reduction in the need for antihypertensive medications at one year compared to the tacrolimus-steroids group (RR 0.19; 95% CI 0.08 to 0.46; p = 0.0002; figure 2D).
Conclusion: This study suggests that a steroid-free regimen with basiliximab is a safe alternative to traditional steroid-based immunosuppression in pediatric liver transplant patients, offering equivalent survival rates while reducing the need for antihypertensive medications one year post-transplant and not increasing infection risk.
References:
[1] Graft Rejection
[2] Basiliximab
[3] Steroid-free regimen
[4] Liver transplant outcomes
[5] Meta-analysis
[6] Immunosuppression
