Postdoc
Interdisciplinary Experimental Transplantation Medicine
Hannover Medical School
The role of endothelial cell senescence in protection from ischemia reperfusion injury
Marco Schmidt1, Khaoula Talbi1, Martin Jaros1, Roland Schmitt2, Anette Melk1.
1Interdisciplinary Experimental Transplantation Medicine, Hannover Medical School, Hannover, Germany; 2Department of Nephrology and Hypertension, University Hospital Schleswig-Holstein, Kiel, Germany
Introduction: Senotherapies are gaining more attention in recent years. Therapeutic approaches in many ongoing clinical trials also offer potential in the context of transplantation. Senotherapies are directed against senescent cells, which have lost the ability to replicate and thereby regenerate. The cell cycle inhibitor p16INK4a is involved in replication-dependent and stress-induced growth arrest. In previous work, we studied p16INK4a in mouse models of ischemia reperfusion injury (IRI) and kidney transplantation (KTx). The absence of p16INK4a resulted in less interstitial fibrosis and tubular atrophy (IF/TA), improved kidney function and better survival after KTx. Similarly, higher p16INK4a expression in human KTx was associated with more IF/TA and inferior graft survival. While senotherapeutic approaches affect all senescent cells, it is still unclear, if the removal of all or just one specific cell type is responsible for the improved outcome. Our aim was therefore to selectively target p16INK4a in endothelial cells to understand the underlying pathomechanisms and potentially reduce off-target-effects of senolytic treatments in the future.
Methods: We generated conditional knockout mice, which are inducible by tamoxifen and driven by the cadherin-5 (VECad) promoter to selectively knockout p16INK4a in endothelial cells. We collected samples at different time points after IRI from young tamoxifen- (TAM) and vehicle (VEH)-injected mice. Two samples from day 3 were selected to generate the first pilot data using single-nuclei RNA sequencing (snRNA-seq) on the 10x Chromium Single Cell GEX 3' platform. In order to validate our data, we additionally reanalyzed publicly available snRNA-seq data from IRI experiments after 4 hours, 12 hours, 2 days, 14 days and 6 weeks performed in WT mice as well as healthy control mice (n = 3 each).
Results: For the snRNA-seq analysis we performed clustering and annotation for all data sets; Fig. 1A illustrates the distribution 3 days after IRI from our pilot samples. When we integrated our VEH control sample with the data from the publicly available dataset, we found comparable results to the public dataset from 2 days after IRI.
Comparison between the TAM- and VEH samples revealed that ablation of p16INK4a in endothelial cells results in fewer injured cells in the kidney. Fig. 1B shows the comparison of the relative cell fractions between both samples. The TAM sample shows fewer nuclei in the injured and failed repair proximal tubule cluster (PT-Inj), while the segment 3 cluster is almost absent in the VEH control.
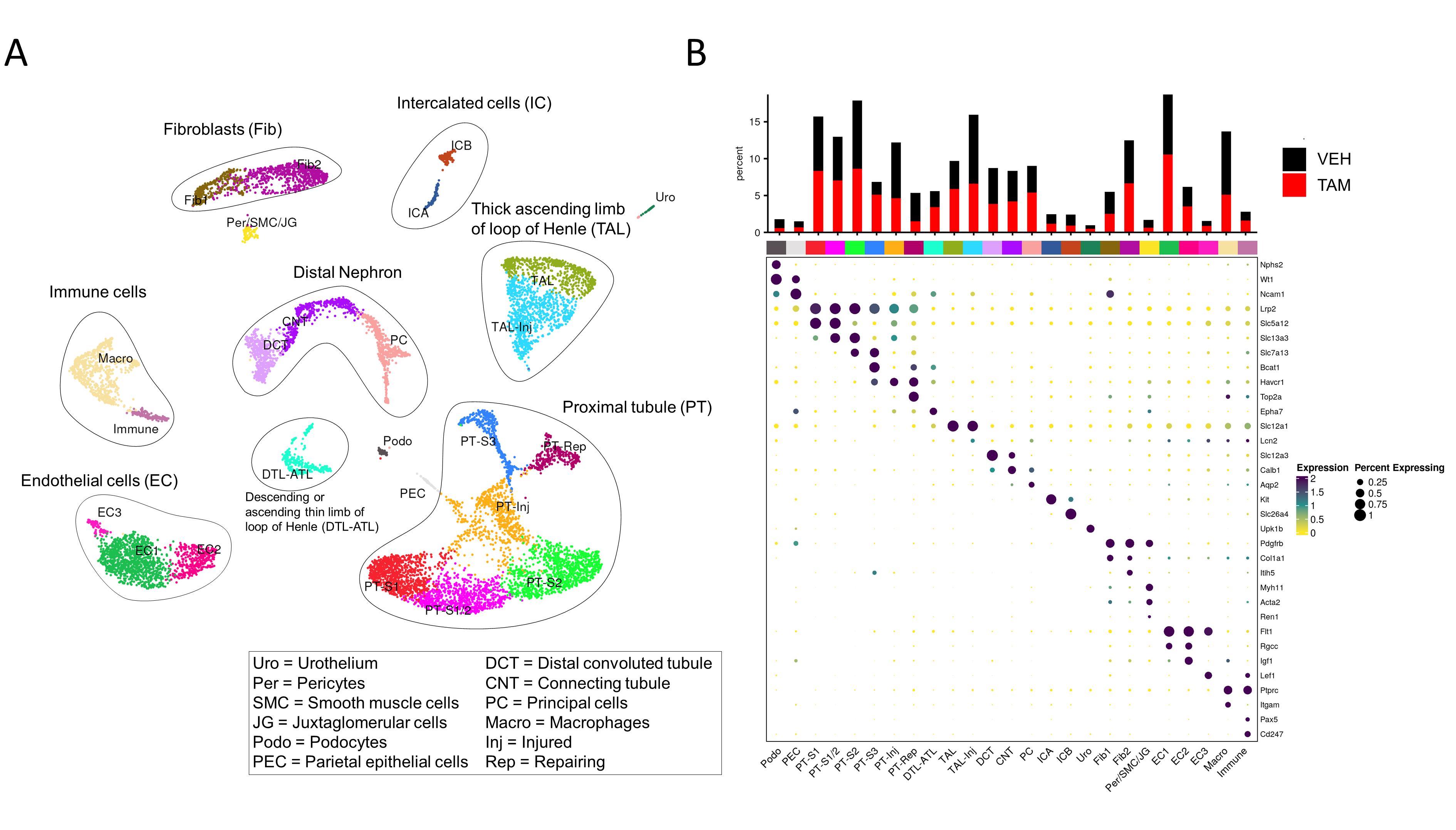
Conclusion: Loss of p16INK4a in endothelial cells resulted in a protection of the kidney already three days after IRI. As increases in p16INK4a expression after IRI usually become apparent at later time points, this early protection was surprising. Therefore, we are currently exploring even earlier time points to understand the underlying pathomechanisms.
This research is supported by the DFG (ME 3696/5-1).
References:
[1] Kidney
[2] Ischemia Reperfusion Injury
[3] Senescence
[4] Single nucleus RNA sequencing
[5] p16
[6] Endothelial cells
Lectures by Marco Schmidt
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Thu-18 16:00 - 17:00 |
Best Kidney Abstracts | The role of endothelial cell senescence in protection from ischemia reperfusion injury | MOA 6 |
