Taihua Yang, People's Republic of China
Shanghai Renji Hospital
Preclinical evaluation of AGT mRNA replacement therapy for primary hyperoxaluria type I disease
Jiahao Ge1, Taihua Yang1, Da-li Li2, Qiang Xia1,3,4.
1Department of Liver Surgery, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, People's Republic of China; 2Shanghai Frontiers Science Center of Genome Editing and Cell Therapy, Institute of Biomedical Sciences and School of Life Sciences, East China Normal University, Shanghai, People's Republic of China; 3/, Shanghai Institute of Organ Transplantation, Shanghai, People's Republic of China; 4/, Shanghai Organ Transplantation and Immune Engineering Technology Research Center, Shanghai, People's Republic of China
Introduction: Primary hyperoxaluria type 1 (PH1) is a rare inherited liver disorder caused by alanine glyoxylate aminotransferase (AGT) dysfunction, leading to accumulation of glyoxylate which is then converted into oxalate. Excessive oxalate results in kidney damage due to deposition of oxalate crystals. Clinically, PH1 typically manifests as recurrent kidney stones, nephrocalcinosis, and chronic kidney disease, potentially progressing to end-stage renal disease (ESRD) if untreated.
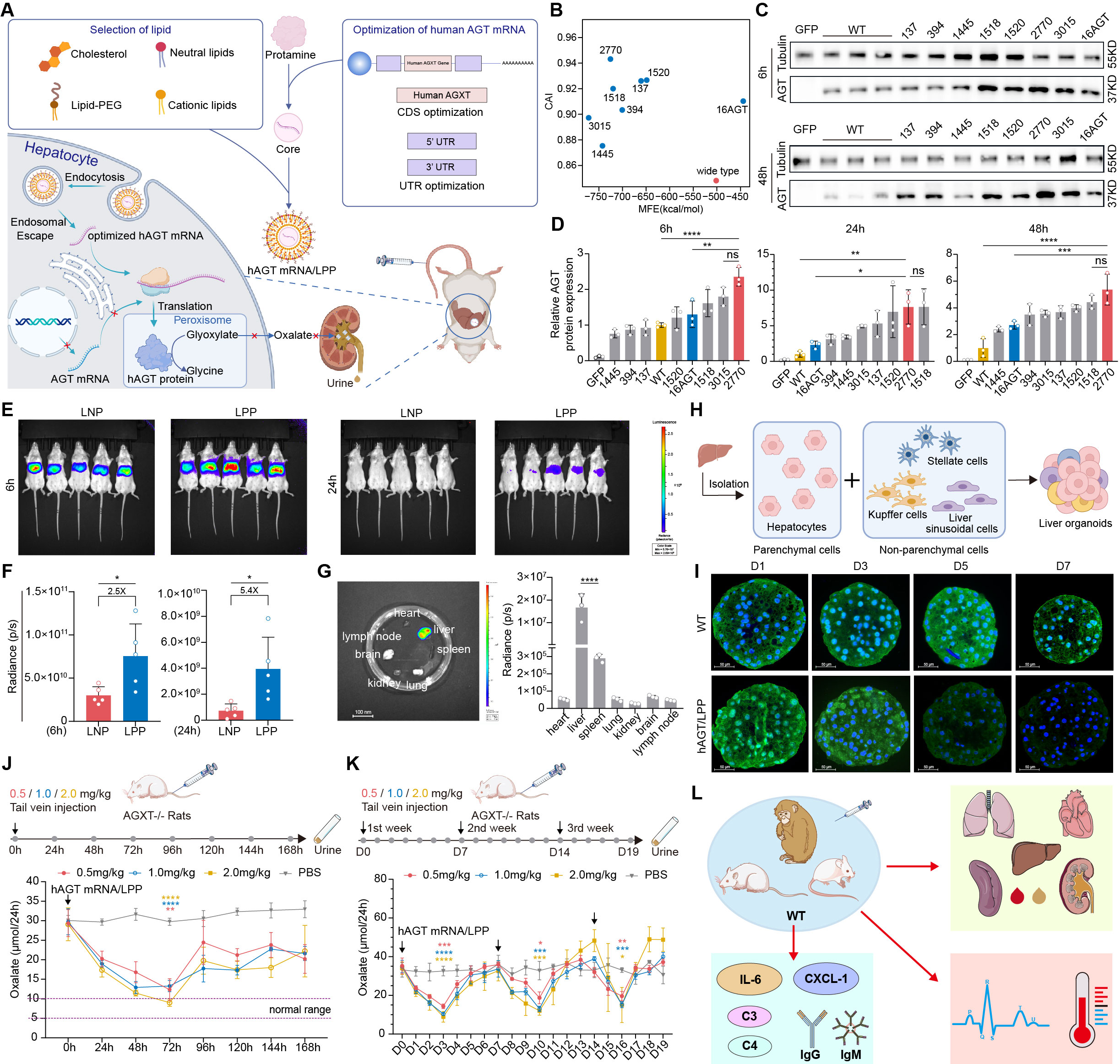
Methods: We have developed an mRNA-based protein replacement therapy for PH1 to restore normal glyoxylate to glycine metabolism. Sequence-optimized human AGT mRNA (hAGT mRNA) was encapsulated in lipopolyplex (LPP) and produced functional AGT enzyme in peroxisomes (A). Pharmacokinetics and pharmacodynamics (PK/PD) were evaluated in vitro and in vivo along with the safety.
Results: The optimized human AGT mRNA exhibited the highest and most sustained protein expression (B-D). The LPP also demonstrated more persistent mRNA expression (E-F) and effective liver targeting (G). Successful construction of human PH1 liver 3D models, followed by AGT mRNA/LPP transfection, demonstrated a preliminary therapeutic effect (H). PK demonstrated that AGT mRNA and protein maintained high expression levels for up to 48 hours (I). A single 2 mg/kg dose in AgxtQ84-/- rats achieved a 70% reduction in urinary oxalate (J). The combination of AGT mRNA/LPP and oral vitamin B6 had a better effect on reducing oxalate concentration. Also, AGT mRNA/LPP rapidly reduced the urinary oxalate to the baseline value in PH1 rat models with acute onset. Furthermore, weekly multiple doses consistently reduced urinary oxalate in PH1 rats (K). Toxicological assessment in rodents and primates identified the highest non-serious toxic dose (HNSTD) as 2 mg/kg (L).
Conclusion: By combining mRNA design with the LPP delivery system we successfully prolonged the duration of protein expression and reduced urine oxalate levels in various PH1 disease animal models. The mRNA/LPP system demonstrated safety and tolerance in both, rodents and primates. Our pre-clinical experiments thus show promising therapeutic potential for mRNA protein replacement therapy in PH1 patients.
Nucleic Acid Drug Project of the National Biopharmaceutical Technology Innovation Center grant NCTIB2022HS02004 . Shanghai Municipal Health Commission Health Industry Clinical Research Project grant 20204Y0351 . Shanghai Science and Technology Innovation Action Plan for 2021 grant No. 21410750400 . National Natural Science Foundation of China grant No. 82100694. National Natural Science Foundation of China grant No. 82300658 . China Postdoctoral Science Foundation grant 2021M702180 . Shanghai Science and Technology Development Funds grant 22YF1423900 . National Science Fund for Distinguished Young Scholars of China grant No. 32025023.
References:
[1] mRNA replacement therapy
[2] urinary oxalate
[3] Primary Hyperoxaluria Type I
Lectures by Taihua Yang
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Thu-18 16:00 - 17:00 |
Best Kidney Abstracts | Preclinical evaluation of AGT mRNA replacement therapy for primary hyperoxaluria type I disease | MOA 6 |
